Abstract
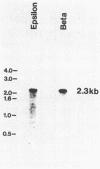
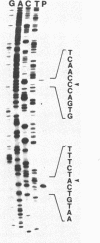
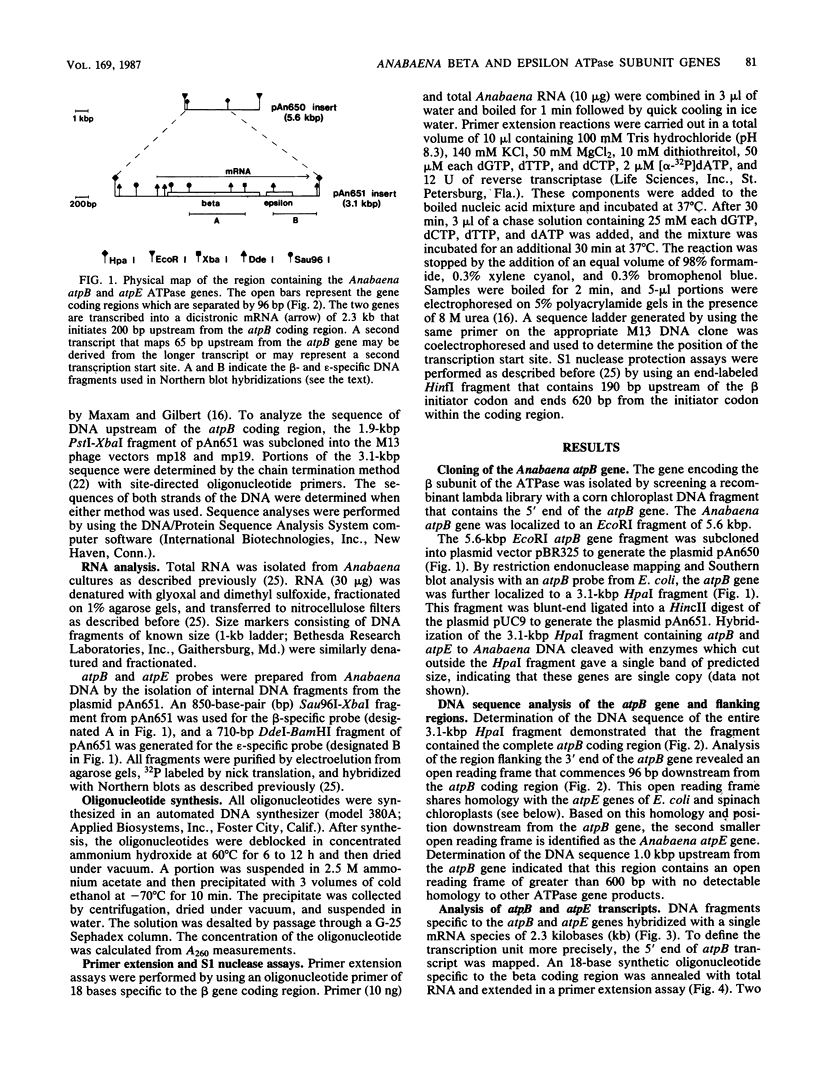
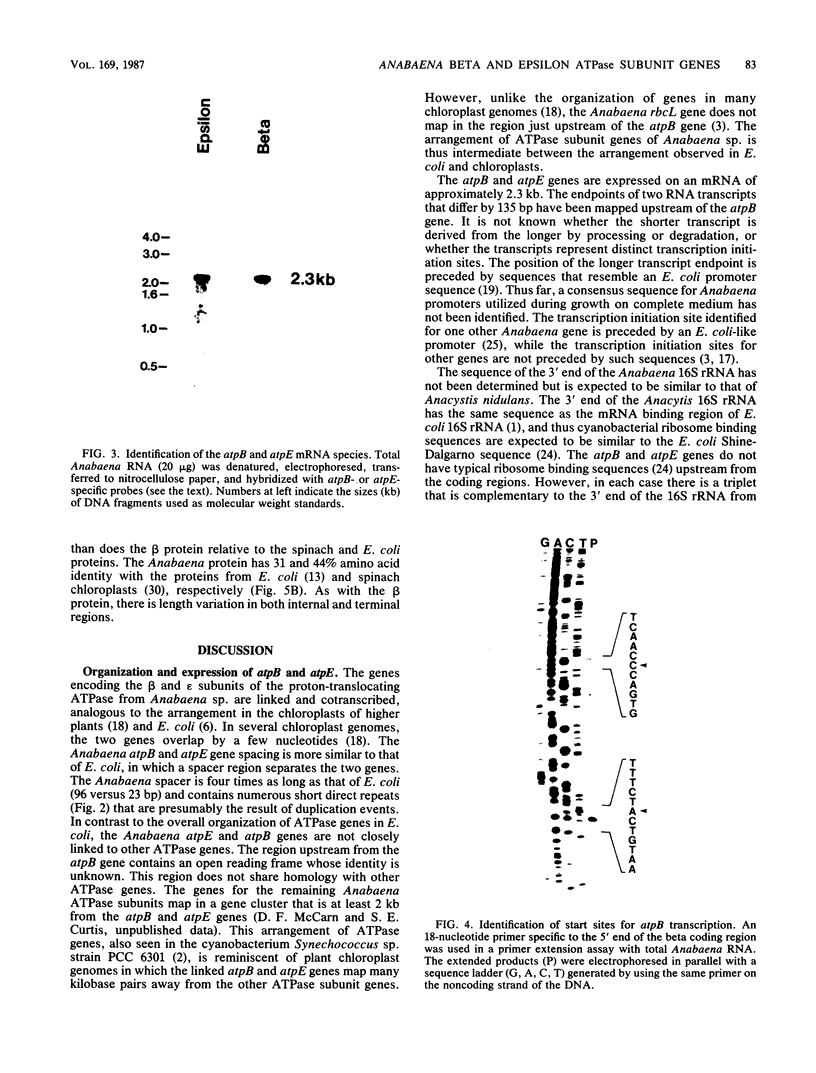
The genes encoding the beta (atpB) and epsilon (atpE) subunits of the ATPase from the cyanobacterium Anabaena sp. strain PCC 7120 were cloned, and their sequences were determined. atpB and atpE are each single-copy genes in the Anabaena genome. The two genes are separated by a 96-base-pair intergenic spacer and transcribed as a single mRNA of 2.3 kilobases that initiates approximately 200 base pairs upstream of the atpB coding region. The predicted translation product of atpB has 81 and 68% amino acid identity with the corresponding proteins from spinach chloroplasts and Escherichia coli, respectively. The atpE gene product is less conserved, with 41 and 33% amino acid identity with the corresponding proteins from spinach chloroplasts and E. coli, respectively. The organization of the Anabaena atpB and atpE genes relative to adjacent genes differs from that of both E. coli and chloroplasts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borbely G., Simoncsits A. 3'-Terminal conserved loops of 16S rRNAs from the cyanobacterium Synechococcus AN PCC 6301 and maize chloroplast differ only in two bases. Biochem Biophys Res Commun. 1981 Aug 14;101(3):846–852. doi: 10.1016/0006-291x(81)91827-1. [DOI] [PubMed] [Google Scholar]
- Cozens A. L., Walker J. E., Phillips A. L., Huttly A. K., Gray J. C. A sixth subunit of ATP synthase, an F(0) component, is encoded in the pea chloroplast genome. EMBO J. 1986 Feb;5(2):217–222. doi: 10.1002/j.1460-2075.1986.tb04201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. E., Haselkorn R. Isolation and sequence of the gene for the large subunit of ribulose-1,5-bisphosphate carboxylase from the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1835–1839. doi: 10.1073/pnas.80.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F. S., Böhlen P., Otsuka A. S., Yoshida M., Allison W. S. Inactivation of the bovine mitochondrial F1-ATPase with dicyclohexyl[14C]carbodiimide leads to the modification of a specific glutamic acid residue in the beta subunit. J Biol Chem. 1981 Sep 10;256(17):9084–9089. [PubMed] [Google Scholar]
- Futai M., Kanazawa H. Structure and function of proton-translocating adenosine triphosphatase (F0F1): biochemical and molecular biological approaches. Microbiol Rev. 1983 Sep;47(3):285–312. doi: 10.1128/mr.47.3.285-312.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby A. A., Castleton J. A., Saul M. W. Expression in E. coli of maize and wheat chloroplast genes for large subunit of ribulose bisphosphate carboxylase. Nature. 1981 May 14;291(5811):117–121. doi: 10.1038/291117a0. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Brusilow W. S., Simoni R. D. Gene order and gene-polypeptide relationships of the proton-translocating ATPase operon (unc) of Escherichia coli. Proc Natl Acad Sci U S A. 1982 Jan;79(2):320–324. doi: 10.1073/pnas.79.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks D. B., Yocum C. F. Properties of the cyanobacterial coupling factor ATPase from Spirulina platensis. I. Electrophoretic characterization and reconstitution of photophosphorylation. Arch Biochem Biophys. 1986 Feb 15;245(1):220–229. doi: 10.1016/0003-9861(86)90208-0. [DOI] [PubMed] [Google Scholar]
- Hicks D. B., Yocum C. F. Properties of the cyanobacterial coupling factor ATPase from Spirulina platensis. II. Activity of the purified and membrane-bound enzymes. Arch Biochem Biophys. 1986 Feb 15;245(1):230–237. doi: 10.1016/0003-9861(86)90209-2. [DOI] [PubMed] [Google Scholar]
- Hollemans M., Runswick M. J., Fearnley I. M., Walker J. E. The sites of labeling of the beta-subunit of bovine mitochondrial F1-ATPase with 8-azido-ATP. J Biol Chem. 1983 Aug 10;258(15):9307–9313. [PubMed] [Google Scholar]
- Kanazawa H., Kayano T., Kiyasu T., Futai M. Nucleotide sequence of the genes for beta and epsilon subunits of proton-translocating ATPase from Escherichia coli. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1257–1264. doi: 10.1016/0006-291x(82)90922-6. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Brusilow W. S., Simoni R. D. In vivo evidence for the role of the epsilon subunit as an inhibitor of the proton-translocating ATPase of Escherichia coli. J Bacteriol. 1984 Dec;160(3):1055–1060. doi: 10.1128/jb.160.3.1055-1060.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nierzwicki-Bauer S. A., Curtis S. E., Haselkorn R. Cotranscription of genes encoding the small and large subunits of ribulose-1,5-bisphosphate carboxylase in the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5961–5965. doi: 10.1073/pnas.81.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. D. Comparative organization of chloroplast genomes. Annu Rev Genet. 1985;19:325–354. doi: 10.1146/annurev.ge.19.120185.001545. [DOI] [PubMed] [Google Scholar]
- Reznikoff W. S., Siegele D. A., Cowing D. W., Gross C. A. The regulation of transcription initiation in bacteria. Annu Rev Genet. 1985;19:355–387. doi: 10.1146/annurev.ge.19.120185.002035. [DOI] [PubMed] [Google Scholar]
- Rice D., Mazur B. J., Haselkorn R. Isolation and physical mapping of nitrogen fixation genes from the cyanobacterium Anabaena 7120. J Biol Chem. 1982 Nov 10;257(21):13157–13163. [PubMed] [Google Scholar]
- Richter M. L., Patrie W. J., McCarty R. E. Preparation of the epsilon subunit and epsilon subunit-deficient chloroplast coupling factor 1 in reconstitutively active forms. J Biol Chem. 1984 Jun 25;259(12):7371–7373. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Gay N. J., Eberle A., Runswick M. J., Walker J. E. The atp operon: nucleotide sequence of the genes for the gamma, beta, and epsilon subunits of Escherichia coli ATP synthase. Nucleic Acids Res. 1981 Oct 24;9(20):5287–5296. doi: 10.1093/nar/9.20.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Eberle A., Gay N. J., Runswick M. J., Saraste M. Conservation of structure in proton-translocating ATPases of Escherichia coli and mitochondria. Biochem Soc Trans. 1982 Aug;10(4):203–206. doi: 10.1042/bst0100203. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Allison W. S., Esch F. S., Futai M. The specificity of carboxyl group modification during the inactivation of the Escherichia coli F1-ATPase with dicyclohexyl[14C]carbodiimide. J Biol Chem. 1982 Sep 10;257(17):10033–10037. [PubMed] [Google Scholar]
- Yoshida M., Poser J. W., Allison W. S., Esch F. S. Identification of an essential glutamic acid residue in the beta subunit of the adenosine triphosphatase from the thermophilic bacterium PS3. J Biol Chem. 1981 Jan 10;256(1):148–153. [PubMed] [Google Scholar]
- Zurawski G., Bottomley W., Whitfeld P. R. Structures of the genes for the beta and epsilon subunits of spinach chloroplast ATPase indicate a dicistronic mRNA and an overlapping translation stop/start signal. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6260–6264. doi: 10.1073/pnas.79.20.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]