Abstract
Gastrointestinal disorders, especially strangulating intestinal obstructions, are still a major cause of illness and death in the horse. Circulating lipopolysaccharides may activate both neutrophils and monocytes. The activated neutrophils release myeloperoxidase (MPO), a specific enzyme with strong oxidative activity. The aim of this study was to evaluate MPO concentrations in the plasma and peritoneal fluid (PF) of horses with colic and to check the hypothesis that these concentrations would be higher in a case of strangulating obstruction than in cases of nonstrangulating disease. By using a specific enzyme-linked immunosorbent assay for equine MPO, we determined the MPO concentrations in horses admitted to a clinic for colic. Horses with nonstrangulating or strangulating obstruction of the large intestine (NSLI or SLI), strangulating obstruction of the small intestine (SSI), or inflammatory bowel disease (IBD) were compared with healthy horses. The horses with SLI, SSI, or IBD had significantly higher MPO levels in plasma and PF than did those in the other 2 groups. The mean plasma level was significantly higher in the horses with NSLI than in the healthy horses. High MPO values in PF indicated necrotic bowel. These results show that neutrophil activation occurs during nonstrangulating and strangulating intestinal obstruction in horses and that the plasma and PF MPO concentrations may be a marker of the severity of the disease.
Résumé
Les pathologies intestinales du cheval, en particulier les obstructions étranglées, sont toujours une cause majeure de morbidité et de mortalité chez le cheval. A côté de l’activation des monocytes via les lipopolysaccharides, une activation des neutrophiles se produit également. Le neutrophile activé relargue la myéloperoxidase (MPO), une enzyme spécifique qui a une activité oxidative puissante. Le but de l’étude est d’évaluer le taux de MPO du plasma et du liquide péritonéal chez des chevaux en coliques à l’admission. L’hypothèse est que les taux de MPO sont plus importants en cas d’obstruction étranglée par rapport à l’obstruction simple. En utilisant un test de dosage immunoenzymatique spécifique de la MPO équine, les auteurs ont déterminé les taux de MPO dans le plasma et le liquide péritonéal des chevaux admis pour colique en clinique. Quatre groupes de chevaux ont été étudiés et comparés aux chevaux sains : des chevaux avec obstruction non-étranglée ou étranglée du gros intestin, obstruction étranglée de l’intestin grêle, et pathologie intestinale de type inflammatoire. Les chevaux avec pathologie étranglée et inflammatoire présentent des taux de MPO du plasma et du liquide péritonéal significativement plus élevés que les chevaux avec pathologie non-étranglée et les sains. Les valeurs de MPO plasmatique sont significativement plus élevées chez les chevaux avec pathologie non-étranglée par rapport aux chevaux sains. Des valeurs élevées de MPO dans le liquide péritonéal sont des indices de nécrose intestinale. Ces résultats montrent l’existence d’une activation des neutrophiles lors des pathologies intestinales étranglées et non-étranglées chez le cheval. La concentration de MPO dans le plasma et le liquide péritonéal peut être un indicateur de la gravité de la pathologie intestinale chez le cheval.
(Traduit par les auteurs)
Introduction
Colic remains a major cause of illness and death in the horse (1,2). Acute abdominal disease can lead to the sequestration of fluid in the obstructed bowel, inducing hypovolemic shock. Strangulation obstruction of the small or large intestine will disrupt the mucosal barrier, favoring lipopolysaccharide leakage into the general circulation and resulting in endotoxemia (3,4).
Circulating lipopolysaccharides can activate neutrophils (5,6) or stimulate monocytes to release mediators contributing to the development of endotoxic shock. Ischemia–reperfusion phenomena in the intestine may exacerbate the cellular injury, with the consequence of excessive neutrophil accumulation and activation (7). With excessive intravascular neutrophil activation, injury to organs other than the compromised bowel may occur, leading to organ dysfunction and failure (8), which explains the high mortality rate.
The stimulation of polymorphonuclear neutrophils results in a sudden increase in oxygen consumption, with the production of reactive oxygen species and the release of enzymes such as proteases (e.g., elastase) and myeloperoxidase (MPO) (9), a hemic enzyme located in the azurophilic granules of the neutrophils. In addition to its peroxidase activity, MPO catalyzes the production of hypochlorous acid (HOCl), a strong oxidant, which contributes to bacterial destruction inside the phagolysosome of the neutrophil (9). Increased plasmatic MPO levels are a marker of neutrophil activation and degranulation in humans (9,10).
There are only a few studies of neutrophil activation in blood and MPO activity in the intestinal tissue of horses with naturally occurring or experimentally induced acute abdominal disease (11,12). To our knowledge, no study has been conducted to date on the MPO concentration in the plasma and peritoneal fluid (PF) of horses with various types of acute abdominal disease. The aim of this work was to evaluate the MPO concentration in the systemic circulation and in the PF of horses with colic by means of a recently designed enzyme-linked immunosorbent assay (ELISA). The values would be compared with those obtained from healthy horses and related to disease process and outcome. The hypothesis was that horses with strangulation obstruction or inflammatory bowel disease (IBD) would have higher MPO levels in plasma and PF than horses with nonstrangulating disease.
Materials and methods
Horses
Included in the study were 103 horses admitted for acute abdominal disease to the Equine Clinic of the Faculty of Veterinary Medicine, University of Liège, Belgium, from December 2002 to June 2005. Clinical data were obtained from a review of the medical records. Only horses with gastrointestinal lesions were included. Demographic data such as age, sex, and breed were recorded for each horse. All the horses underwent a clinical and rectal examination as well as nasogastric intubation on admission. Blood was drawn to determine packed cell volume, blood gas levels, leukocyte count, and biochemical profile. In 42 cases the clinical condition also permitted the sampling of sufficient PF for analysis. After examination, the horses were treated medically or surgically by ventral midline celiotomy, or euthanasia was performed (because of poor prognosis or because no surgery was permitted for financial reasons) and a necropsy done. The outcome of hospitalization was categorized as survival or nonsurvival. Horses euthanized for financial reasons were excluded from the survival analysis. The affected portion of the gastrointestinal tract and the disease process (obstruction or tympany, strangulation obstruction, IBD, or peritonitis) were identified. The final diagnosis was categorized as nonstrangulating large intestinal lesion (NSLI, group 1), strangulating large intestinal lesion (SLI, group 2), strangulating small intestinal lesion (SSI, group 3), or inflammatory bowel disease (IBD, group 4), which included various conditions, such as typhlocolitis, proximal enteritis, and peritonitis.
For a comparison group of heathy horses, we used a control group of 38 horses whose plasma MPO values had been determined in an earlier study (13). All belonged to the herd of the veterinary faculty and the center for locomotion research at Mont-le-Soie, Belgium, and had been found to be healthy by physical examination. We obtained PF from 17 of the horses: 10 warmblood and 7 draught horses aged 2 to 19 y; 7 were females, 3 males, and 7 geldings.
Blood and PF sampling technique
Blood samples were collected into tubes containing ethylene diamine tetraacetic acid (EDTA) upon admission of the horses to the clinic and immediately centrifuged for 10 min at 1000 × g. The plasma was stored at −20°C until the MPO assay. The PF was sampled by needle or cannula puncture at the lowest point of the abdomen, near the ventral midline. In the presence of abdominal distension, ultrasonography was used to locate the PF. It was collected into tubes containing EDTA and centrifuged for 10 min at 1000 × g. Small aliquots of the supernatant were stored at −20°C until the MPO assay.
Assay of MPO in plasma and PF
The centrifuged plasma and PF samples were diluted 40 times in 20 mM phosphate-buffered saline (pH 7.4) containing 5 g/L of bovine serum albumin and 0.1% Tween 20. The MPO concentrations were determined, with reference to a standard curve, by means of an original ELISA, as previously described (13), with the use of 2 specific polyclonal antibodies obtained from rabbit (primary antibody) and guinea pig (secondary antibody). The “sandwich complex” primary antibody–MPO–secondary antibody was recognized by a goat antibody to guinea pig immunoglobulin coupled to alkaline phosphatase. Phosphatase activity was detected by incubation (for 30 min at 37°C in the dark) with a paranitrophenyl phosphate solution (phosphatase substrate). The reaction was stopped with 2.5 M NaOH and the absorbance at 405 nm read with the Multiscan Ascent spectrophotometer (Thermo Labsystems, Helsinki, Finland).
The standard curve was established with known concentrations of purified equine MPO ranging from 0.78 to 50 ng/mL. The assay had previously been validated with equine blood and plasma (13,14).
Statistical analysis
All data were obtained in triplicate, and results of calculations are reported as means (and standard deviation). The previously obtained mean plasma concentration for healthy horses was 181.8 (64.74) ng/mL (13). A maximum physiological value of 376 ng/mL (mean + 3 standard deviations) was determined to ensure a probability of error lower than 0.01% in determining abnormally high values. For each group the percentage of horses that showed MPO values above the maximum was determined.
The data were analyzed by Kruskal–Wallis nonparametric analysis of variance with Dunn’s multiple-comparison post-test (comparison of mean plasma MPO values between the 4 disease groups and the control group). For comparisons of only 2 groups, we used an unpaired t test with the Welch correction (comparison of mean plasma MPO values between horses with or without shock signs and between survivors and nonsurvivors, as well as comparison of mean PF MPO values between the control group and groups 2, 3, and 4 together). The relation between plasma MPO value and total leukocyte count was tested by determining the Pearson correlation coefficient (r). A P-value of less than 0.05 was considered significant. All statistical analyses were performed with the GraphPad InStat 3.05 program (GraphPad Software, San Diego California, USA).
Results
Among the 103 horses admitted to the clinic, 70 (68%) were of the warmblood breed, a frequency similar to that in the general hospital population. The other horses consisted of 13 ponies, 5 Appaloosas, 4 thoroughbreds, 4 draught horses, 3 Arabians, 2 standardbreds, and 2 Andalusians. There were 49 castrated males (48%), 48 mares (47%), and 6 sexually intact males (6%). Their age ranged from 1 to 27 y, with a mean of 10.6 (6.1) y and a median of 9 y. Their weight varied from 251 to 790 kg. Although 56 horses survived to discharge, 47 horses were euthanized after admission owing to severe shock signs or reasons other than medical, during surgery because the lesions were inoperable or too extensive, or during the postoperative phase. The 14 horses euthanized for nonmedical reasons were excluded from the survival analysis.
Of the 38 horses with an NSLI (group 1), 14 had displacement in the renosplenic space, 13 impaction colic of the pelvic flexure, 7 other displacements of the ascending colon, and 4 cecal tympany. Of the 21 horses with an SLI (group 2), 18 had complete large colon torsion or volvulus, and 1 horse each had cecal torsion, cecocolic intussusception, or strangulating lipoma of the descending colon. Of the 32 horses with an SSI, 17 had incarcerated jejunum or ileum in the epiploic foramen, 7 jejunal volvulus, 5 a strangulating lipoma, 1 a diaphragmatic hernia, 1 ileocecal intussusception, and 1 ileal impaction with jejunal volvulus. Of the 12 horses with various inflammatory or infectious bowel and peritoneal diseases (group 4), 5 had typhlocolitis (combined with proximal enteritis in 1 horse), 4 proximal enteritis, and 3 peritonitis.
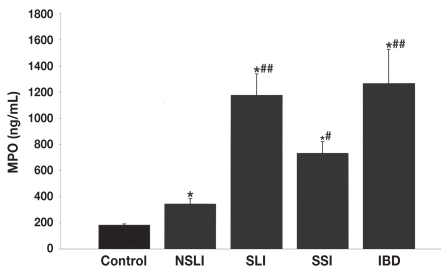
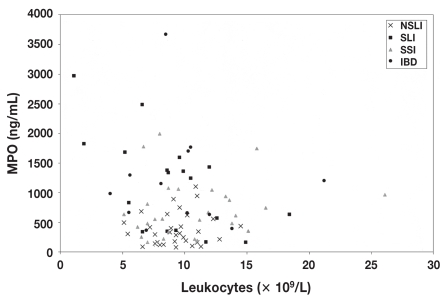
The mean plasma MPO value was 343.16 (260.81) ng/mL (range 76 to 1099 ng/mL) for the horses with an NSLI, significantly different (2-tailed P = 0.0006; Welch’s approximate t = 3.702 with 41 degrees of freedom [df]) from the mean for the healthy controls, 181.8 (64.74) ng/mL. For the horses in groups 2, 3, and 4, the mean plasma MPO values were even higher, at 1176.38 (755.74) ng/mL (range 567.12 to 2975.20 ng/mL), 733.28 (505.36) ng/mL (range 164 to 1993 ng/mL), and 1206.8 (906.97) ng/mL (range 365 to 3670 ng/mL), respectively, and were significantly higher than the means for the healthy controls and group 1 (P < 0.01) (Figure 1). Plasma MPO values above the physiological maximum of 376 ng/mL were obtained for 34%, 76%, 75%, and 92% of the horses in groups 1 to 4, respectively. No correlation was observed between the total blood leukocyte count and the MPO value in any of the groups evaluated (Figure 2).
Figure 1.
Mean plasma concentrations of myeloperoxidase (MPO) (and standard deviation) in the control group of healthy horses and 4 groups of horses with gastrointestinal disease. NSLI — nonstrangulating obstruction of the large intestine; SLI — strangulating obstruction of the large intestine; SSI — strangulating obstruction of the small intestine; IBD — inflammatory bowel disease. Asterisks indicate a significant difference from the control mean, at P < 0.05, and number signs a significant difference from the NSLI mean, at P < 0.001 for the SLI and IBD means and P < 0.01 for the SSI mean.
Figure 2.
Lack of correlation between the plasma MPO value and the total blood leukocyte count (× 109/L) in the 4 groups of horses with gastrointestinal disease: r = −0.1485 (not significant).
In the NSLI group, only 2 horses (5%) showed signs of endotoxic shock, whereas 13 (62%) of the SLI group and 19 (59%) of the SSI group showed signs of shock. In all horses showing signs of endotoxic shock, the plasma MPO values were above the maximum physiological value. The mean MPO value was 379.04 (291.28) ng/mL in the 57 horses without signs of shock, whereas it was 1236.4 (759.72) ng/mL in the 35 horses with signs of shock (P < 0.0001).
Survival analysis was performed with groups 1 to 3 but without the 14 horses that were euthanized because financial constraints precluded surgery or enterectomy. Of the remaining 77 horses, 47 survived to discharge. The mean plasma MPO value was significantly higher (P < 0.0001) in the nonsurvivor group than in the survivor group, at 1199.23 (644.87) versus 391 (295.78) ng/mL. In the nonsurvivor group, all horses except 1 had plasma MPO values above the physiological maximum, whereas only 18 of the horses in the survivor group had such values.
The mean MPO value obtained with 17 PF samples from healthy horses was 338.66 (178.66) ng/mL (range 101.84 to 761.16 ng/mL). For the horses in groups 1 to 4 whose PF was sampled, the mean MPO values were 181 (112.18) ng/mL (range 74 to 339 ng/mL) for the 4 horses with NSLI, 1193.6 (818.08) ng/mL (range 88 to 2178 ng/mL) for the 5 horses with SLI, 1169.46 (955.99) ng/mL (range 107 to 2893 ng/mL) for the 24 horses with SSI, and 1137.78 (686.01) ng/mL (range 72 to 2204 ng/mL) for the 9 horses with IBD. Table I shows the plasma and PF MPO values of these 42 horses, as well as respective pathological and clinical details.
Table I.
Pathological and clinical details, as well as plasma and peritoneal fluid (PF) concentrations of myeloperoxidase (MPO), for 42 horses with gastrointestinal disease
| MPO level (ng/mL)
|
|||||
|---|---|---|---|---|---|
| Diseasea | Lived | Shockb | Necrosisc | Plasma | PF |
| NSLI | |||||
| RS entrapment | Yes | – | – | 175 | 339 |
| Impaction | Yes | – | – | 188 | 74 |
| Cecal tympany | Yes | – | – | 396 | 160 |
| Cecal tympany | Yes | – | – | 331 | 151 |
| SLI | |||||
| CC intuss. | No | – | +++ | 830 | 1464 |
| ACT | No | +++ | +++ | 1359 | 1567 |
| ACT | No | ++ | +++ | 1825 | 2178 |
| ACT | No | +++ | +++ | 1431 | 671 |
| ACT | Yes | – | – | 339 | 88 |
| SSI | |||||
| IEF | Yes | – | ++ | 218 | 107 |
| IEF | Yes | – | ++ | 335 | 484 |
| IEF | No | +++ | +++ | 871 | 2365 |
| IEF | No | +++ | +++ | 963 | 2390 |
| IEF | No | – | +++ | 478 | 2748 |
| IEF | No | ++ | ++ | 1895 | 505 |
| IEF | No | +++ | +++ | 806 | 2893 |
| IEF | No | – | ++ | 1075 | 867 |
| IEF | No | +++ | +++ | 479 | 714 |
| IEF | No | ++ | ++ | 935 | 443 |
| IEF | No | – | ++ | 188 | 251 |
| IEF | No | – | ++ | 213 | 774 |
| IEF | No | ++ | ++ | 352 | 489 |
| IEF | No | +++ | +++ | 1743 | 2177 |
| S lipoma | Yes | – | +++ | 253 | 883 |
| S lipoma | No | – | +++ | 423 | 477 |
| S lipoma | No | ++ | +++ | 611 | 2048 |
| S lipoma | No | +++ | +++ | 1993 | 2580 |
| S lipoma | No | +++ | +++ | 1778 | 2355 |
| Volvulus | No | ++ | ++ | 665 | 531 |
| Volvulus | No | ++ | ++ | 654 | 209 |
| Volvulus | No | – | ++ | 221 | 418 |
| Ileal imp., volvulus | Yes | – | – | 1059 | 371 |
| D hernia | No | +++ | +++ | 1044 | 988 |
| IBD | |||||
| P enteritis | Yes | – | NA | 628 | 72 |
| P enteritis | Yes | ++ | NA | 3670 | 477 |
| P enteritis | Yes | ++ | NA | 365 | 1892 |
| Typhlocolitis | Yes | – | NA | 1152 | 958 |
| Typhlocolitis | No | +++ | NA | 1297 | 807 |
| Peritonitis | No | +++ | NA | 393 | 1031 |
| Peritonitis | Yes | ++ | NA | 1203 | 2204 |
| Peritonitis | Yes | +++ | NA | 1767 | 1713 |
| Colitis, enteritis | No | ++ | NA | 982 | 1086 |
NSLI — nonstrangulating obstruction of the large intestine; RS — renosplenic; SLI — strangulating obstruction of the large intestine; CC intuss. — cecocolic intussusception; ACT — ascending colon torsion; SSI — strangulating obstruction of the small intestine; IEF — incarceration of the jejunum and/or ileum into the epiploic foramen; S — strangulating; imp. — impaction; D — diaphragmatic; IBD — inflammatory bowel disease; P — proximal.
Signs of endotoxic shock: none (−), moderate (++), or severe (+++).
Intestinal necrosis: none (−), moderate (++), severe (+++), or not applicable (NA).
Owing to the low numbers of PF samples in the large-intestine groups, we amalgamated the data for the 4 groups and obtained a mean of 1071.4 (869.7) ng/mL for these 42 samples, which was significantly higher (2-tailed P < 0.0001; Welch’s approximate t = 5.122 with 50 df) than the mean for the control horses.
When we compared the horses of the SSI group having severe intestinal necrosis (n = 12) with the horses without or with moderate vascular lesions (no enterectomy necessary; n = 10), a significant difference in MPO content was found (P < 0.0002; Welch’s approximate t = 5.423 with 12 df). In 8 of the 12 horses, the MPO values in PF were particularly high. The mean MPO concentration in the PF of the horses with severe necrosis was 1884.8 (864.68) ng/mL, whereas it was 485.8 (206.06) ng/mL for the horses with moderate vascular lesions.
Discussion
The study reported here reveals that neutrophil activation occurs in a high percentage of horses with intestinal obstruction, especially strangulating obstruction, and that MPO is released into the plasma as well as the PF. Likewise, in a prior study, horses with SLI showed the highest plasma MPO values (15). In cases of experimental low-flow ischemia of the large colon, accumulation of neutrophils in the colonic mucosa as well as increased MPO concentrations have previously been demonstrated (7,16). The large colon contains more endotoxin than the small intestine. The toxin enters the systemic circulation owing to permeability changes during strangulating obstruction and can directly activate the neutrophils (5). The large number of resident neutrophils in the colonic mucosa can also contribute to the higher MPO values. Furthermore, we observed increased MPO values in IBD, confirming the recent observation of increased serum amyloid A, an acute-phase protein, in horses with IBD (17). More cases should be evaluated to confirm whether animals with IBD show higher MPO values than those with strangulating lesions. This could be an interesting new element in differentiating proximal enteritis from small intestinal obstruction.
In our study, horses with NSLI, especially those with large- intestine displacement and severe tympany, had higher plasma MPO values than healthy horses. This finding is in contrast to the lack of observed neutrophil activation in nonstrangulated colic cases in another study (12). Sequestration of fluid and severe intestinal distension can lead to hypovolemic shock and could activate neutrophils, resulting in an increase in MPO concentration.
Owing to the high variability of MPO levels, we could not establish a cut-off point to distinguish survivors from nonsurvivors. A larger study should be conducted. Additionally, postoperative follow-up could show that in some cases MPO values return to normal rapidly in surviving horses, as has been shown previously (15).
Studies of the prognostic and diagnostic value of PF in acute abdominal disease have shown that color and specific gravity or total protein concentration are more valuable than nucleated cell count for establishing prognosis or lesion type (18,19). In our study, the presence of high MPO levels in the PF in strangulating obstruction indicated necrotic bowel and a poor prognosis. Interestingly, the MPO level is not correlated with the neutrophil count. Activated neutrophils may degranulate and disappear rapidly, so that only the proteases are left. High PF MPO content is more indicative of SSI than is a high plasma MPO level. Similarly, a recent study showed that the PF lactate level was a better predictor of a strangulating lesion than was the plasma lactate level (20).
Recent studies have shown the implication of neutrophil activation in noninfectious diseases such as paralytic ileus (21) and laminitis (22,23) occurring as complications of strangulating obstruction in horses. Indeed, excessive neutrophil activation and degranulation could release active MPO as well as other proteases into the blood stream or tissues. Beyond its antimicrobial activity, MPO shows cytotoxicity towards erythrocytes and endothelial cells (24,25). It can also lead to platelet activation and enzyme inactivation. By inactivating antiproteases and activating proteases, MPO may also disturb the coagulation and protease/antiprotease balance (26). This could contribute to the development of postoperative complications. Another method is being developed to assay active MPO specifically (27). In this way, the implication of MPO in various pathological mechanisms could be defined. Inhibitors of MPO could be developed to modulate inflammation.
In conclusion, equine intestinal diseases are linked to the activation of neutrophils in the systemic circulation as well as in the peritoneal cavity. The MPO assay could therefore be useful in predicting the severity of the disease.
Acknowledgments
We thank Mrs. Yvette Goutman and Josiane Neuray for their excellent technical assistance and the Centre Européen du Cheval de Mont-le-Soie for the blood reference samples. This work was supported by a research grant (011/4695 MPOEQUI) from the Région Wallonne: Programme de Recherche Initiative.
References
- 1.Cohen ND, Matejka PL, Honnas CM, Hooper RN. Case–control study of the association between various management factors and development of colic in horses. Texas Equine Colic Study Group. J Am Vet Med Assoc. 1995;206:667–673. [PubMed] [Google Scholar]
- 2.Tinker MK, White NA, Lessard P, et al. Prospective study of equine colic risk factors. Equine Vet J. 1997;29:454–458. doi: 10.1111/j.2042-3306.1997.tb03158.x. [DOI] [PubMed] [Google Scholar]
- 3.Moore JN, White NA, Berg JN, Trim CM, Garner HE. Endotoxemia following experimental intestinal strangulation obstruction in ponies. Can J Comp Med. 1981;45:330–332. [PMC free article] [PubMed] [Google Scholar]
- 4.King J, Gerring E. Detection of endotoxin in cases of equine colic. Vet Rec. 1988;123:269–271. doi: 10.1136/vr.123.10.269. [DOI] [PubMed] [Google Scholar]
- 5.Benbarek H, Deby-Dupont G, Caudron I, et al. Interaction between lipopolysaccharides and blood factors on the stimulation of equine polymorphonuclear neutrophils. Vet Immunol Immunopathol. 1998;64:313–322. doi: 10.1016/s0165-2427(98)00142-1. [DOI] [PubMed] [Google Scholar]
- 6.Dagleish MR, Brazil TJ, Scudamore CL. Potentiation of the extracellular release of equine neutrophil elastase and alpha-1-proteinase inhibitor by a combination of two bacterial cell wall components: fMLP and LPS. Equine Vet J. 2003;35:35–39. doi: 10.2746/042516403775467496. [DOI] [PubMed] [Google Scholar]
- 7.Moore RM, Bertone AL, Bailey MQ, Muir WW, Beard WL. Neutrophil accumulation in the large colon of horses during low-flow ischemia and reperfusion. Am J Vet Res. 1994;55:454–463. [PubMed] [Google Scholar]
- 8.Partrick DA, Moore FA, Moore EE, Barnett CC, Jr, Silliman CC. Neutrophil priming and activation in the pathogenesis of post-injury multiple organ failure. New Horiz. 1996;4:194–210. [PubMed] [Google Scholar]
- 9.Deby-Dupont G, Deby C, Lamy M. Neutrophil myeloperoxidase revisited: its role in health and disease. Intensivmed Notfallmed. 1999;36:500–513. [Google Scholar]
- 10.Hoy A, Leininger-Muller B, Kutter D, Siest G, Visvikis S. Growing significance of myeloperoxidase in non-infectious diseases. Clin Chem Lab Med. 2002;40:2–8. doi: 10.1515/CCLM.2002.002. [DOI] [PubMed] [Google Scholar]
- 11.McConnico RS, Weinstock D, Poston ME, Roberts MC. Myeloperoxidase activity of the large intestine in an equine model of acute colitis. Am J Vet Res. 1999;60:807–813. [PubMed] [Google Scholar]
- 12.Weiss DJ, Evanson OA. Evaluation of activated neutrophils in the blood of horses with colic. Am J Vet Res. 2003;64:364–1368. doi: 10.2460/ajvr.2003.64.1364. [DOI] [PubMed] [Google Scholar]
- 13.Franck T, Grulke S, Deby-Dupont G, et al. Design and development of an enzyme-linked immunosorbent assay for specific equine neutrophil myeloperoxidase measurement in blood. J Vet Diagn Invest. 2005;17:412–419. doi: 10.1177/104063870501700502. [DOI] [PubMed] [Google Scholar]
- 14.Deby-Dupont G, Grulke S, Caudron I, et al. Equine myeloperoxidase in plasma: design of a radio-immunoassay and first results in septic pathologies. Vet Immunol Immunopathol. 1998;66:257–271. doi: 10.1016/s0165-2427(98)00192-5. [DOI] [PubMed] [Google Scholar]
- 15.Grulke S, Benbarek H, Caudron I, et al. Plasma myeloperoxidase level and polymorphonuclear leukocyte activation in horses suffering from large intestinal obstruction requiring surgery: preliminary results. Can J Vet Res. 1999;63:142–147. [PMC free article] [PubMed] [Google Scholar]
- 16.Yarbrough B, Snyder JR, Harmon FA, O’Connell KA. Evaluation of myeloperoxidase concentrations in experimentally induced equine colonic ischaemia and reperfusion. Equine Vet J. 1994;26:67–69. doi: 10.1111/j.2042-3306.1994.tb04334.x. [DOI] [PubMed] [Google Scholar]
- 17.Vandenplas ML, Moore JN, Barton MH, Roussel AJ, Cohen ND. Concentrations of serum amyloid A and lipopolysaccharide-binding protein in horses with colic. Am J Vet Res. 2005;66:1509–1516. doi: 10.2460/ajvr.2005.66.1509. [DOI] [PubMed] [Google Scholar]
- 18.Freden GO, Provost PJ, Rand WM. Reliability of using results of abdominal fluid analysis to determine treatment and predict lesion type and outcome for horses with colic: 218 cases (1991–1994) J Am Vet Med Assoc. 1998;213:1012–1015. [PubMed] [Google Scholar]
- 19.Matthews S, Dart AJ, Reid SW, Dowling BA, Hodgson DR. Predictive values, sensitivity and specificity of abdominal fluid variables in determining the need for surgery in horses with an acute abdominal crisis. Aust Vet J. 2002;80:132–136. doi: 10.1111/j.1751-0813.2002.tb11372.x. [DOI] [PubMed] [Google Scholar]
- 20.Latson KM, Nieto JE, Beldomenico PM, Snyder JR. Evaluation of peritoneal fluid lactate as a marker of intestinal ischaemia in equine colic. Equine Vet J. 2005;37:342–346. doi: 10.2746/0425164054529319. [DOI] [PubMed] [Google Scholar]
- 21.Little D, Tomlinson JE, Blikslager AT. Post operative neutrophilic inflammation in equine small intestine after manipulation and ischaemia. Equine Vet J. 2005;37:329–335. doi: 10.2746/0425164054529472. [DOI] [PubMed] [Google Scholar]
- 22.Hurley DH, Parks RJ, Reber AJ, et al. Dynamic changes in circulating leukocytes during the induction of equine laminitis with black walnut extract. Vet Immunol Immunopathol. 2006;110:195–206. doi: 10.1016/j.vetimm.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Riggs LM, Franck T, Moore JN, et al. Neutrophil myeloperoxidase measurements in plasma, laminar tissue, and skin of horses given black walnut extract. Am J Vet Res. 2007;68:81–86. doi: 10.2460/ajvr.68.1.81. [DOI] [PubMed] [Google Scholar]
- 24.Tobler A, Koeffler HP. Myeloperoxidase: localization, structure, and function. In: Harris GR, editor. Blood Cell Biochemistry. 3. Lymphocytes and Granulocytes. New York: Plenum Press; 1991. pp. 255–288. [Google Scholar]
- 25.Mathy-Hartert M, Deby-Dupont G, Deby C, Jadoul L, Vandenberghe A, Lamy M. Cytotoxicity induced by neutrophil myeloperoxidase towards human endothelial cells: protection by ceftazidime. Mediators Inflamm. 1995;4:1–7. doi: 10.1155/S0962935195000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suter PM, Suter S, Girardin E, Roux-Lombard P, Grau GE, Dayer JM. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am Rev Respir Dis. 1992;145:1016–1022. doi: 10.1164/ajrccm/145.5.1016. [DOI] [PubMed] [Google Scholar]
- 27.Franck T, Kohnen S, Deby-Dupont G, Grulke S, Deby C, Serteyn D. A specific method for measurement of equine active myeloperoxidase in biological samples and in in vitro tests. J Vet Diagn Invest. 2006;18:326–334. doi: 10.1177/104063870601800402. [DOI] [PubMed] [Google Scholar]