Abstract
The objective of this research was to evaluate reactivation of bovine viral diarrhea virus (BVDV) following dexamethasone treatment in 4 bulls that had previously been inoculated with BVDV, 3 of which had been demonstrated to have a localized testicular infection. Bulls were housed in an isolated pasture with in-contact steers. Beginning on day 0 of this study, all bulls received a daily dose of 0.1 mg/kg body weight (BW) of dexamethasone intravenously for 5 consecutive days. Blood was collected from the in-contact steers and semen, blood, and cerebrospinal fluid were collected from the bulls during and following dexamethasone treatment. Samples were assayed for BVDV using virus isolation and reverse transcription-nested polymerase chain reaction (RT-nPCR). Serum was assayed for antibody using standard virus isolation. Virus was not isolated from blood, cerebrospinal fluid, or semen from any of the 4 bulls during the study period. One of the bulls was positive for BVDV in semen by RT-nPCR throughout the study period. The BVDV was not recovered from any in-contact control steers during the 28-day study period, nor did any of the in-contact control steers seroconvert to BVDV. Raw semen from 1 bull that was RT-nPCR positive was intravenously inoculated into 7 seronegative steers based upon the Cornell Semen Test. The BVDV could not be recovered from the steers and none of them seroconverted to BVDV. The results indicated that reactivation of BVDV in bulls with a localized testicular infection is unlikely; however, further research is necessary to determine the full potential for BVDV transmission from bulls with a localized testicular infection.
Résumé
L’objectif du projet était d’évaluer la réactivation du virus de la diarrhée virale bovine (BVDV) suite à un traitement à la dexaméthasone chez 4 taureaux qui avaient précédemment été inoculés avec du BVDV, et parmi lesquels 3 avaient présenté une infection testiculaire localisée. Les taureaux étaient logés dans un pré avec des bouvillons contacts. À partir du jour 0 de l’étude, tous les taureaux ont reçu pendant 5 jours consécutifs une dose quotidienne de dexaméthasone par voie intraveineuse à raison de 0,1 mg/kg de poids corporel (BW). Du sang a été prélevé chez les bouvillons contacts et de la semence, du sang et du liquide céphalo-rachidien ont été prélevés à partir des taureaux pendant et après le traitement à la dexaméthasone. Les échantillons ont été éprouvés pour la présence du BVDV par isolement viral et réaction d’amplification en chaîne par la polymérase nichée à l’aide de la transcriptase réverse (RT-nPCR). Les échantillons de sérum ont été testés pour la présence d’anticorps en utilisant l’isolement viral standard. Aucun virus ne fut isolé du sang, du liquide céphalo-rachidien ou de la semence des 4 taureaux au cours de la période d’étude. Un des taureaux s’est avéré positif pour le BVDV dans la semence par RT-nPCR pour toute la durée de l’étude. Durant la période d’étude de 28 jours, le BVDV ne fut isolé d’aucun des bouvillons contacts, et aucun de ces animaux n’a présenté de séroconversion au BVDV. De la semence non-traitée provenant du taureau positif par RT-nPCR a été injectée par voie intraveineuse à 7 bouvillons séronégatifs selon le «Cornell Semen Test». Le BVDV n’a pas été ré-isolé à partir des bouvillons et aucun n’a présenté de séroconversion au BVDV. Les résultats indiquent que la réactivation du BVDV chez des taureaux avec une infection testiculaire localisée est peu probable; toutefois, des recherches supplémentaires sont nécessaires afin de déterminer le plein potentiel d’une transmission du BVDV à partir de taureaux avec une infection testiculaire localisée.
(Traduit par Docteur Serge Messier)
Introduction
Bovine viral diarrhea virus (BVDV), a major cattle pathogen, has a global distribution, and is responsible for a wide spectrum of clinical manifestations, including reproductive failure, and respiratory and gastrointestinal tract diseases (1). Two types of infection, acute or persistent, may occur with BVDV. Infection of susceptible pregnant cattle with noncytopathic BVDV during the first 125 d of gestation may result in the birth of a calf immunotolerant to and persistently infected with BVDV (2). Acute BVDV infections in immunocompetent cattle occur with cytopathic or noncytopathic BVDV. Cattle persistently infected with BVDV are considered to be the reservoir of the virus and the major source of spread within and between cattle populations (3). In contrast, acutely infected animals are viremic briefly, and thus have not been considered efficient transmitters of BVDV (4).
Bulls acutely and persistently infected with BVDV are capable of shedding virus in semen (5–8). Bulls persistently infected with BVDV shed large quantities of virus [107,.6 cell culture infectious dose 50%/mL (107,.6 CCID50/mL)] in the seminal fluid; BVDV may survive cryopreservation and processing of semen for artificial insemination and thus could infect susceptible female cattle (9, 10). In general, acutely infected bulls shed lower concentrations of BVDV in semen (5–75 CCID50/mL) (9); however, infection of artificially inseminated heifers may occur using semen from acutely infected bulls prior to seroconversion (7). Although acute BVDV infections generally result in a transient viremia with subsequent clearance of the virus by the host immune system, a localized infection of testicular tissue with noncytopathic BVDV has been described under both natural and experimental conditions (6, 11). The original description of localized testicular infection with BVDV was identified in the testes of a seropositive, nonviremic bull at an artificial insemination center (11). Despite absence of viremia, the bull continuously shed infectious BVDV in semen throughout his life. Virus in the semen of this bull resulted in infection and subsequent seroconversion of an inseminated seronegative heifer (12). Furthermore, this bull displayed a consistently high concentration of circulating serum antibodies that neutralized the specific viral strain that was persistently shed in the semen. After slaughter, virus could only be isolated from the testes of this animal (12). Since that original description, localized testicular infections with BVDV have been reproduced in postpubertal bulls, and virus has persisted within testicular tissue of some bulls for at least 7 mo following experimental inoculation (6). For months after acute infection, BVDV was detected in semen by reverse transcription-nested polymerase chain reaction (RT-nPCR) but could not be isolated. Despite this inability to isolate virus, BVDV in semen collected 5 mo after inoculation proved to be infectious when administered by the intravenous route to a seronegative calf (6).
There is uncertainty about whether bulls with a localized testicular infection may become viremic and infectious to other animals following stress. In 1996, 26.8% of cow/calf beef operations in the United States indicated that a new weaned bull was brought onto their operation (13). Since bulls are the most common class of cattle that beef operations purchase, lease, or borrow, it is important to assess whether bulls with a localized testicular infection can become viremic and shed BVDV. Dexamethasone is commonly used to pharmacologically mimic some of the effects of the elevated activity of the hypothalamic-pituitary-adrenocortical axis that accompanies stress (14). The purpose of this study was to evaluate reactivation of BVDV following dexamethasone treatment in 4 bulls that had previously been inoculated with BVDV, 3 of which had been demonstrated to have a localized testicular infection.
Materials and methods
Animals and dexamethasone treatment
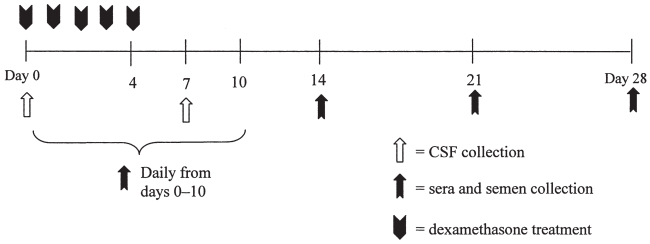
Four, approximately 400-kg postpubertal bulls that had been previously inoculated with a noncytopathic BVDV-1a isolate were used in this study. All bulls had been inoculated by intranasal aerosol administration with 5.0 mL of cell culture supernatant that contained 105 CCID50/mL of the noncytopathic BVDV-1a strain SD-1(15). The present study was initiated 237 days after the experimental inoculation of the bulls. Three bulls (A, B, and C) had been demonstrated to have a localized testicular infection with BVDV SD-1 as defined by positive RT-nPCR results on semen (Table I). The 4th bull (control; D) was a bull previously exposed to the aforementioned BVDV-1a SD-1 strain, but this bull did not develop a localized testicular infection. Beginning on day 0 of this study, all bulls received a daily dose of 0.1 mg/kg body weight of dexamethasone by intravenous injection for 5 consecutive days (Figure 1) (16, 17). Semen, blood, and cerebrospinal fluid were collected during and following dexamethasone treatment.
Table I.
RT-nPCR results for semen on bulls
| Bull ID | Daya 93 | Day 135 | Day 150 | Day 181 | Dayb 237 | Day 282 | Day 373 |
|---|---|---|---|---|---|---|---|
| A | POS | POS | POS | POS | POS | POS | POS |
| B | POS | POS | NEG | NEG | NEG | NEG | ND |
| C | POS | POS | POS | NEG | NEG | NEG | ND |
| D | NEG | NEG | NEG | NEG | NEG | NEG | ND |
Denotes day after experimental inoculation with noncytopathic BVDV-1a isolate SD-1.
Day 237 corresponds to day 0 of the present study.
ND — not determined.
Figure 1.
Experimental design.
Four, 6-month-old steers were used as in-contact, sentinel controls during the 28-day study period. These steers were determined to be seronegative to BVDV by virus neutralization and negative for BVDV by virus isolation. The steers were added to the isolated pastures with the bulls 5 days prior to beginning the study. All bulls and steers were isolated from all other livestock for the duration of the study at Auburn University. The experiment was performed with approval and under the guidelines of the Auburn University Institutional Animal Use and Care Committee (Auburn University, IACUC No. 2004–0753).
Clinical examination and sample collection
Blood and semen samples were collected, and clinical examinations, including rectal temperature measurements, were performed on the bulls on days 0 to 10, 14, 21, and 28 after the initiation of dexamethasone treatment (Figure 1). Blood samples were collected and clinical examinations performed on the in-contact control steers on days 0, 6 to 10, 14, 21, and 28. Serum was separated from clotted blood and stored at −80°C. Whole blood collected in ethylene diamine tetraacetic acid (EDTA) was processed to yield the buffy coat cells. Following centrifugation of the whole blood at 200 × g for 30 min, the buffy coat cells were removed. Lysis of contaminating red blood cells was performed using 0.15 M ammonium chloride (NH4Cl). The buffy coat cells were washed in 10 mL of minimum essential medium (MEM) containing 10% (vol:vol) equine serum (ES), sodium bicarbonate (0.75 mg/mL), L-glutamine (0.29 mg/mL), penicillin G (100 IU/mL), streptomycin (100 μg/mL), and amphotericin B (0.25 μg/mL). After centrifugation at 300 × g for 10 min, the buffy coat cells were resuspended in 0.5 mL of medium to be used in virus isolation procedures.
Cerebrospinal fluid was collected from the lumbosacral space of the bulls on days 0 and 7 after commencing dexamethasone treatment. Following administration of a local anesthetic, a 15.2-cm, 18-gauge spinal needle was used to collect cerebrospinal fluid by inserting the needle through the skin and advancing it until the tip punctured the lumbosacral cistern. The cerebrospinal fluid was then stored at −80°C.
Semen was collected by use of an electroejaculator. An aliquot of raw semen was partially extended (1:8) in egg-yolk citrate extender (6). Raw and extended semen samples were stored at −80°C.
Virus isolation from serum, cerebrospinal fluid, buffy coat cells, and semen
Serum samples and cerebrospinal fluid were assayed for BVDV by passage through Madin Darby bovine kidney (MDBK) cells. A 6-well plate that had been seeded 24 h earlier with MDBK cells in MEM containing 10% ES was inoculated with 768 μL of serum or cerebrospinal fluid diluted in 192 μL of media. Following a 1-h adsorption period, 3 mL of MEM with 10% ES was added. The plates were incubated for 5 days. Following a single freeze-thaw cycle to release intracellular virus, lysates from this procedure were assayed in triplicate by diluting 10 μL of cell lysate with 90 μL of culture medium and subsequently adding 50 μL of culture medium containing MDBK cells to the wells of a 96-well culture plate. Following incubation for 72 h at 37°C in humidified air containing 5% CO2, the MDBK cells were stained for BVDV antigen by an immunoperoxidase monolayer assay using the BVDV-specific monoclonal antibodies D89 and 20.10.6 (6).
Detection of BVDV in buffy coat cells was performed through co-cultivation with MDBK cells. Briefly, the 0.5-mL suspension of buffy coat cells was layered over MDBK cells in 0.5 mL of medium that had been previously seeded into wells of a 24-well culture plate 24 h earlier. Following a 1-h adsorption period, 3 mL of MEM with 10% ES was added. The plates were incubated for 4 days. Following a single freeze-thaw cycle to release intracellular virus, lysates from this procedure were assayed in triplicate using the immunoperoxidase monolayer assay procedure as described for serum and cerebrospinal fluid.
Frozen, partially extended semen samples were thawed, and an initial 1:10 dilution was made by diluting 100 μL of the samples into 900 μL of culture medium. This initial dilution was performed to prevent cytotoxicity. A 24-well plate that had been seeded 24 h earlier with MDBK cells in 900 μL MEM containing 10% ES was inoculated with 100 μL of the diluted semen suspension. Plates were incubated for 5 days. Following a single freeze-thaw cycle to release intracellular virus, lysates from this procedure were assayed in triplicate using the immunoperoxidase monolayer assay procedure as described for serum.
RT-nPCR, real-time RT-PCR, and sequencing of the RT-PCR products
Serum, semen, and cerebrospinal fluid samples were assayed for BVDV using a single closed-tube reverse transcriptase-nested polymerase chain reaction (RT-nPCR) procedure according to previously described methods (18). A single closed-tube reaction system was used to prevent carry-over contamination, and primers were selected to amplify a sequence of the 5′ nontranslated region of the BVDV genome. The outer primers, BVDV 100 (5′-GCTAGCCATGCCCTTAG-3′) and HCV 368 (5′-CCATGTGCCATGTACAG-3′) amplified a 290 base pair sequence. The inner primers, BVD 180 (5′-CCTGAGTACAGG GDAGT CGTCA-3′) and HCV 368 amplified a 213 base pair sequence within the first amplicon. All RT-nPCR products were compared to known negative and positive control samples using submarine gel electrophoresis and ultraviolet transillumination of ethidium bromide stained 1.5% agarose gels.
When the RT-nPCR procedure yielded a positive result, a real-time, quantitative RT-PCR procedure was performed. Nucleic acids for detection of BVDV by quantitative RT-PCR were isolated as previously described for RT-nPCR. Ribonucleic acid samples were stored in 60 μL of elution buffer at −80°C until performance of quantitative RT-PCR. All real-time PCRs were performed in duplicate reactions in volumes of 15 μL reaction master mixture and 5 μL sample aliquot in LightCycler glass capillaries in a Roche LightCycler real-time thermocycler (Roche Diagnostics, Indianapolis, Indiana, USA). The PCR buffer was 4.5 mM magnesium chloride (MgCl2), 50 mM potassium chloride (KCl), 20 mM tris-hydrogen chloride (tris-HCL), pH 8.4, supplemented with 0.05% Tween 20 (Sigma Chemical Company, St. Louis, Missouri, USA) and Nonidet P-40 (Roche Applied Science, Indianapolis, Indiana, USA) and 0.03% acetylated bovine serum albumin (Roche Applied Science). Nucleotides (Promega, Madison, Wisconsin, USA) were used at 0.2 mM [deoxyadenosine triphosphate (dATP, deoxycytidine triphosphate (dCTP), deoxyguanosine triphosphate (dGTP)] and 0.6 mM deoxyuridine triphosphate (dUTP). Each reaction used 0.02 U of Thermoscript reverse transcriptase (Invitrogen, Calsbad, California, USA), 2 U of Taq DNA polymerase (Promega), and 0.2 U of heat-labile uracil-DNA glycosalase (UNG) (Roche Applied Science). Each reaction used 1 μM of L1 and U3 primers that were specific for conserved areas of the 5′ nontranslated region of the viral genome of BVDV(19). Polymerase chain reaction products were detected using hybridization probes s1 (0.1 μM; labeled on the 3′ end with carboxyfluorescein) and s2 (0.2 μM; labeled on the 5′ end with Bodipy 630/650 and synthesized with a 3′ phosphate to block extension by Taq polymerase; HPLC purified) (19). For each real-time PCR, reaction master mixture was freshly assembled from separate stocks of distilled water, 5 × PCR buffer, 5 × oligonucleotides (primers and probes) in TE buffer, 50 × PCR Nucleotide MixPLUS (Roche Applied Science), Taq DNA polymerase, uracil-DNA glycosylase (UNG; Roche Applied Science), and Thermoscript reverse transcriptase (Invitrogen). The RT-PCR reaction included a reverse transcription step at 55°C for 30 min; denaturation at 95°C for 2 min; 6 cycles at 95°C for 1 s, 60°C for 12 s, and 72°C for 11 s; 9 cycles at 95°C for 1 s; 58°C for 12 s, and 72°C for 11 s; and 3 cycles at 95°C for 1 s, 56°C for 12 s, and 72°C for 11 s. Finally, 40 cycles were performed at 95°C for 1 s, 55°C for 8 s, and 58°C for 11 s with fluorescence acquisition; and 72°C for 11 s. The melting curve of each PCR product was determined to confirm nucleotide sequence homology. Products of PCR were synthesized using deoxyuridine triphosphate (dUTP) instead of deoxythymidine triphosphate (dTTP). Heat-labile UNG was added to PCR reactions to ensure PCR products could not produce false positives due to inadvertent cross-contamination in subsequent reactions. To allow quantitation of PCR products, external standards were constructed by amplifying complementary DNA from RNA extracted using a silica gel-based membrane kit (QIAamp viral RNA Mini Kit; QIAGEN, Valencia, California, USA) from cell culture lysate of the type 1a strain of BVDV (SD-1). This DNA was purified by high-resolution agarose gel electrophoresis and filtration. The DNA was quantified using the PicoGreen DNA fluorescence assay (Molecular Probes, Eugene, Oregon, USA) and used at 104, 103, 102, and 10 genome copies per 5 μL in a background of sheared plasmid DNA in 10 mM tris-HCL (pH 8.5) and 0.1 mM EDTA. In each reaction, a negative control (water), positive control RNA, and external DNA standards were included. Data were displayed and analyzed as 640:530 nm fluorescence ratios.
The RT-nPCR was performed in triplicate on raw semen collected from bull A on day 0. The RT-nPCR products were purified using a silica gel-based membrane kit (QIAamp Viral RNA Mini Kit; QIAGEN) and sequenced by automated dye terminator nucleotide sequencing using both the 5′ and 3′ primers (BVDV 100 and HCV 298, respectively). Consensus sequences were determined using Align X computer software (Vector NTI Suite 7.1; InforMax, Bethesda, Maryland, USA), and compared to the sequence from SD-1, the challenge strain that had been used to experimentally inoculate the bulls.
Virus neutralization
A standard virus neutralization microtiter assay was used for the detection and quantification of antibodies in serum. Sera were tested for neutralizing antibodies to the BVDV-1a strain with which the bulls had been inoculated. Sera were inactivated for 30 minutes in a 56°C water bath. Serial 2-fold dilutions, ranging from 1:2 to 1:2048, were made for each serum sample in 50 μL of culture media. For each dilution, 3 wells of a 96-well microtiter plate were inoculated with 50 μL of culture media containing 100 CCID50 of BVDV-1a isolate SD-1. The plates were incubated for 1 h at 37°C in humidified air containing 5% CO2. The MDBK cells were used as the indicator cells by adding 50 μL of cells per well. Each test included a back titration of the virus and a positive and negative serum control.
Bioassay via inoculation of calves
Seven 6-month-old steers that were determined to be seronegative to BVDV and nonviremic were isolated from all contact with other livestock for the duration of an inoculation experiment that was based on the Cornell Semen Test (20). The objective of this procedure was to evaluate the infectivity of BVDV detected in semen by use of the RT-nPCR technique. One steer served as a noninoculated control, while the remaining 6 steers were inoculated intravenously with raw semen obtained from 2 of the adult bulls. Clinical examinations were performed and blood was collected from steers on days 0 (day of inoculation with raw semen), 6 to 10, and 28. Sera and whole blood were collected and processed for virus neutralization and virus isolation procedures as described previously.
Results
Clinical findings
Pyrexia (> 40°C) was occasionally observed in bulls. On day 0, all bulls had rectal temperatures > 40°C, and 1 bull (D) had an elevated temperature (40.1°C) on day 1 as well. Pyrexia was not observed in bulls from day 2 to 9 of the study, and 2 bulls (B and C) had temperatures > 40°C on day 10. All steers were pyrectic on day 0 of the study, but no elevations in temperature were observed in the steers throughout the remainder of the study.
Beginning on day 7, after initiation of dexamethasone injections, all 4 bulls developed severe hemorrhagic diarrhea. A standard fecal flotation using Sheather’s sucrose solution (specific gravity = 1.27) followed by examination under light microscopy was performed on day 7 on all bulls and steers to search for internal parasites. Heavy infestation with protozoan parasites of the genus Eimeria was noted in the 4 bulls, but not in the 4 steers. Based upon this finding, the hemorrhagic diarrhea was attributed to eimeriosis, and all bulls were treated once daily for 5 days with 62.5 mg/kg BW, PO, of oral sulfadimethoxine (Albon SR; Pfizer Animal Health, Exton, Pennsylvania, USA). By day 10 of the study, feces had returned to normal.
Virus isolation and virus neutralization
Virus was not recovered from sera, buffy coat cells, cerebrospinal fluid, or semen from any of the 4 bulls during the study period. In addition, BVDV was not recovered from any in-contact control steers during the 28-day study period.
None of the in-contact control steers seroconverted to BVDV during the study period, as evidenced by lack of antibody titers against BVDV SD-1 on day 28 of the study.
RT-nPCR and real-time RT-PCR
For bull A, the RT-nPCR assay for semen was positive on every sample day throughout the study period. The other 3 bulls (B, C, and D) were negative by RT-nPCR on all semen samples collected during the study period. Virus could not be detected by RT-nPCR in serum or cerebrospinal fluid from any bulls during the study period.
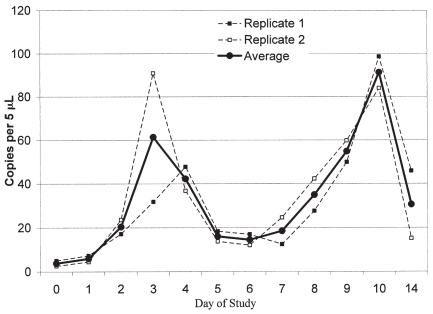
Real-time RT-PCR was performed in duplicate on all semen samples from bull A (Figure 2). Two peaks in the viral RNA concentration were noted. The 1st peak was observed at day 3 and the 2nd peak was observed at day 10 of the study period.
Figure 2.
Results of real time RT-PCR on semen samples for Bull B.
Bioassay via inoculation of calves
Raw semen samples collected from bulls A and C were used to inoculate seronegative steers. One steer served as a noninoculated control, while the remaining 6 steers were inoculated intravenously with raw semen. Of these 6 inoculated steers, 1 had been inoculated with semen from bull C (day 10 of the study), and this semen sample had been determined to be RT-nPCR negative. The remaining 5 steers were inoculated with 5 different RT-nPCR positive raw semen samples from bull A (days 0, 3, 6, 10, or 28).
Clinical examinations were performed and none of the steers exhibited pyrexia or illness. In addition, BVDV could not be detected from these steers by virus isolation from serum or buffy coat cells at any time following inoculation with the raw semen. None of the semen-inoculated steers seroconverted to BVDV during the study period, as evidenced by lack of antibody titers against BVDV SD-1 28 days after inoculation with raw semen.
Discussion
Bovine viral diarrhea virus can be identified in semen from persistently infected bulls, acutely infected bulls prior to seroconversion, and from bulls with a localized testicular infection (6–8). Recrudescence, or reactivation, is a term that has been used primarily for herpes virus infections (16,21); however, this may apply to any pathogen that evades immune clearance and becomes active when conditions are favorable for transmission. The purpose of this novel research was to determine if BVDV can undergo reactivation from testicular tissue following the administration of dexamethasone, similar to that described for latent herpes virus infection (16). Since bulls are the most common class of cattle that beef operations purchase, lease, or borrow, assessing recrudescence of BVDV from bulls with a localized testicular infection is extremely important considering the impact that a potentially infectious bull can have upon reproduction within a beef herd. Additionally, localized testicular infections with BVDV cannot be diagnosed using blood samples or ear notches. If reactivation poses a threat to biosecurity on the farm, testing methods for newly introduced bulls would need to be modified to identify bulls with a localized testicular infection (6, 11). However, results from this study indicate that BVDV did not enter the systemic circulation nor could infectious BVDV be cultured from semen after dexamethasone administration to bulls with a localized testicular infection. Additionally, BVDV was not transmitted through semen after intravenous inoculation of seronegative steers with raw semen. Collectively, these findings suggest that although BVDV can persist after acute infection in semen as identified by RT-nPCR, the potential for these animals to transmit virus following stress is negligible.
The 4 bulls used in this study were chosen based primarily upon availability for study, but also for their differences in historical identification of BVDV in semen by RT-nPCR. Only bull A was RT-nPCR-positive at the onset of study, and 2 other bulls were previously documented to have had a localized testicular infection, but were determined to be negative for BVDV by RT-nPCR on semen at the onset of this study. Inclusion of additional bulls in this study that were RT-nPCR positive in semen would have been ideal; however, bulls that met these criteria were not available. Further study evaluating reactivation following dexamethasone treatment in bulls with a localized BVDV testicular infection is necessary to validate the negligibility of reactivation of BVDV.
Host and viral factors are important determinants for the induction of viremia and clinical disease during BVDV infection. Although the results indicate that dexamethasone treatment did not result in reactivation of BVDV from testicular tissue, the results may have differed if the dexamethasone challenge been initiated earlier in the course of a localized testicular infection, such as day 93 rather than day 237 after experimental inoculation, as done in this study. Other factors that could cause our assessment of negligibility to be incorrect include viral load within the testicle and the magnitude of stress and immunosuppression.
Bull A was positive by RT-nPCR throughout the study period. Interestingly, in follow-up real-time RT-PCR testing, there appeared to have been a biphasic increase in viral RNA concentration in semen that peaked on days 3 and 10 of the study period, respectively. Although speculative, the peak in copy number at day 3 of the study may correspond to the effect of the dexamethasone, while the peak at day 10 of the study may correspond to the effects of coccidiosis. Although these peaks in viral RNA concentration were identified, BVDV could not be cultured from the semen samples at those time points, nor could BVDV be isolated from blood, or seroconversion demonstrated in the steers that had been inoculated with raw semen collected on days 3 and 10 of the study period, thus the relevance of this increase in copy number is questionable.
Other viruses of cattle have been recovered from semen. As reviewed by Wrathal et al (22), these viruses include bovine herpesvirus-1, bovine leukemia virus, bluetongue virus, foot and mouth disease virus, and bovine immunodeficiency virus. In addition, lumpy skin disease virus, a pox virus, has been recently identified in Africa to be excreted in semen (23). The majority of these bovine pathogens are associated with semen in the form of adventitious virus found in the seminal plasma, or in cellular constituents of semen rather than the spermatozoa themselves. Bovine herpes virus-1 recrudescence and shedding in semen is thought to be due primarily to infectious herpes virus in the seminal plasma rather than spermatozoa, although bovine herpes virus-1 antigen has been localized to the spermatozoa (24). In contrast, BVDV antigen can be identified in seminiferous tubules and virus can be cultured from the testicular tissue of bulls with localized testicular infections (6). It has been hypothesized that BVDV becomes localized in the gonadal cells at the period of development of the blood-testes barrier, thus keeping BVDV hidden from the immune response (11). Other immunologically priveleged sites include brain tissue (blood-brain barrier) and the ovaries. Virus has been localized in ovarian tissue for prolonged periods of time following acute BVDV infection (25). We chose to examine cerebrospinal fluid in these bulls with localized testicular infection to examine for the presence of BVDV in a site other than testicular tissue. In small ruminants and cattle, abundant viral antigen has been located in the central nervous system following acute and persistent BVDV infections (26,27). Viral antigen can be found in cerebrum, cerebellum, and spinal cord. Our findings, although in a limited study population, indicate that BVDV is not recovered from cerebrospinal fluid in bulls that also possess a localized testicular infection.
In summary, localized testicular infections with BVDV create concerns about the potential sequelae of venereal transmission, subfertility, or reactivation of a systemic infection during times of stress. This research, involving a limited number of bulls, suggests that although BVDV can persist in semen as identified by RT-nPCR, the potential for these animals to transmit virus following stress is negligible. Further research involving more substantial numbers of bulls with a localized testicular infection is necessary to fully evaluate this potential.
Footnotes
This study was supported by Animal Health Research, Auburn University.
References
- 1.Baker JC. The clinical manifestations of bovine viral diarrhea infection. Vet Clin North Am Food Anim Pract. 1995;11:425–445. doi: 10.1016/s0749-0720(15)30460-6. [DOI] [PubMed] [Google Scholar]
- 2.McClurkin AW, Littledike ET, Cutlip RC, Frank GH, Coria MF, Bolin SR. Production of cattle immunotolerant to bovine viral diarrhea virus. Can J Comp Med. 1984;48:156–161. [PMC free article] [PubMed] [Google Scholar]
- 3.Brock KV. The persistence of bovine viral diarrhea virus. Biologicals. 2003;31:133–135. doi: 10.1016/s1045-1056(03)00029-0. [DOI] [PubMed] [Google Scholar]
- 4.Niskanen R, Lindberg A, Tråvén M. Failure to spread bovine virus diarrhoea virus infection from primarily infected calves despite concurrent infection with bovine coronavirus. Vet J. 2002;163:251–259. doi: 10.1053/tvjl.2001.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyling A, Jensen AM. Transmission of bovine virus diarrhoea virus (BVDV) by artificial insemination (AI) with semen from a persistently-infected bull. Vet Microbiol. 1988;17:97–105. doi: 10.1016/0378-1135(88)90001-6. [DOI] [PubMed] [Google Scholar]
- 6.Givens MD, Heath AM, Brock KV, Brodersen BW, Carson RL, Stringfellow DA. Detection of bovine viral diarrhea virus in semen obtained after inoculation of seronegative postpubertal bulls. Am J Vet Res. 2003;64:428–434. doi: 10.2460/ajvr.2003.64.428. [DOI] [PubMed] [Google Scholar]
- 7.Kirkland PD, McGowan MR, Mackintosh SG, Moyle A. Insemination of cattle with semen from a bull transiently infected with pestivirus. Vet Rec. 1997;140:124–127. doi: 10.1136/vr.140.5.124. [DOI] [PubMed] [Google Scholar]
- 8.Kirkland PD, Mackintosh SG, Moyle A. The outcome of widespread use of semen from a bull persistently infected with pestivirus. Vet Rec. 1994;135:527–529. doi: 10.1136/vr.135.22.527. [DOI] [PubMed] [Google Scholar]
- 9.Kirkland PD, Richards SG, Rothwell JT, Stanley DF. Replication of bovine viral diarrhoea virus in the bovine reproductive tract and excretion of virus in semen during acute and chronic infections. Vet Rec. 1991;128:587–590. doi: 10.1136/vr.128.25.587. [DOI] [PubMed] [Google Scholar]
- 10.McGowan MR, Kirkland PD. Early reproductive loss due to bovine pestivirus infection. Br Vet J. 1995;151:263–270. doi: 10.1016/s0007-1935(95)80176-6. [DOI] [PubMed] [Google Scholar]
- 11.Voges H, Horner GW, Rowe S, Wellenberg GJ. Persistent bovine pestivirus infection localized in the testes of an immunocompetent, non-viraemic bull. Vet Microbiol. 1998;61:165–175. doi: 10.1016/s0378-1135(98)00177-1. [DOI] [PubMed] [Google Scholar]
- 12.Niskanen R, Alenius S, Belak K, et al. Insemination of susceptible heifers with semen from a non-viraemic bull with persistent bovine virus diarrhoea virus infection localized in the testes. Reprod Domest Anim. 2002;37:171–175. doi: 10.1046/j.1439-0531.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- 13.USDA:APHIS:VS. Part II: reference of 1997 beef cow-calf production management practices. Fort Collins, CO: Center for Epidemiology and Animal Health; 1997. [Google Scholar]
- 14.Anderson BH, Watson DL, Colditz IG. The effect of dexamethasone on some immunological parameters in cattle. Vet Res Commun. 1999;23:399–413. doi: 10.1023/a:1006365324335. [DOI] [PubMed] [Google Scholar]
- 15.Deng R, Brock KV. Molecular cloning and nucleotide sequence of a pestivirus genome, noncytopathic bovine viral diarrhea virus strain SD-1. Virology. 1992;191:867–869. doi: 10.1016/0042-6822(92)90262-n. [DOI] [PubMed] [Google Scholar]
- 16.Grom J, Hostnik P, Toplak I, Barlic-Maganja D. Molecular detection of BHV-1 in artificially inoculated semen and in the semen of a latently infected bull treated with dexamethasone. Vet J. 2006;171:539–544. doi: 10.1016/j.tvjl.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Castrucci G, Frigeri F, Osburn BI, Ferrari M, Sawyer MM, Aldrovandi V. A study of some pathogenetic aspects of bovine viral diarrhea virus infection. Arch Virol Suppl. 1991;3:101–108. doi: 10.1007/978-3-7091-9153-8_12. [DOI] [PubMed] [Google Scholar]
- 18.Givens MD, Heath AM, Carson RL, et al. Analytical sensitivity of assays used for detection of bovine viral diarrhea virus in semen samples from the Southeastern United States. Vet Microbiol. 2003;96:145–155. doi: 10.1016/s0378-1135(03)00213-x. [DOI] [PubMed] [Google Scholar]
- 19.Studer E, Bertoni G, Candrian U. Detection and characterization of pestivirus contaminations in human live viral vaccines. Biologicals. 2002;30:289–296. doi: 10.1006/biol.2002.0343. [DOI] [PubMed] [Google Scholar]
- 20.Schultz RD, Adams LS, Letchworth G, Sheffy BE, Manning T, Bean B. A method to test large numbers of bovine semen samples for viral contamination and results of a study using this method. Theriogenology. 1982;17:115–123. doi: 10.1016/0093-691x(82)90071-1. [DOI] [PubMed] [Google Scholar]
- 21.Homan EJ, Easterday BC. Experimental latent and recrudescent bovine herpesvirus-1 infections in calves. Am J Vet Res. 1983;44:309–313. [PubMed] [Google Scholar]
- 22.Wrathall AE, Simmons HA, Van Soom A. Evaluation of risks of viral transmission to recipients of bovine embryos arising from fertilisation with virus-infected semen. Theriogenology. 2006;65:247–274. doi: 10.1016/j.theriogenology.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 23.Irons PC, Tuppurainen ES, Venter EH. Excretion of lumpy skin disease virus in bull semen. Theriogenology. 2005;63:1290–1297. doi: 10.1016/j.theriogenology.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Elazhary MASY, Lamothe P, Silim A, Roy RS. Bovine herpesvirus type 1 in the sperm of a bull from a herd with fertility problems. Can Vet J. 1980;21:336–339. [PMC free article] [PubMed] [Google Scholar]
- 25.Grooms DL, Brock KV, Ward LA. Detection of bovine viral diarrhea virus in the ovaries of cattle acutely infected with bovine viral diarrhea virus. J Vet Diagn Invest. 1998;10:125–129. doi: 10.1177/104063879801000201. [DOI] [PubMed] [Google Scholar]
- 26.Gruber AD, Greiser-Wilke IM, Haas L, Hewicker-Trautwein M, Moennig V. Detection of bovine viral diarrhea virus RNA in formalin-fixed, paraffin-embedded brain tissue by nested polymerase chain reaction. J Virol Methods. 1993;43:309–319. doi: 10.1016/0166-0934(93)90149-l. [DOI] [PubMed] [Google Scholar]
- 27.Wöhrmann T, Hewicker-Trautwein M, Fernandez A, Moennig V, Liess B, Trautwein G. Distribution of bovine virus diarrhoea viral antigens in the central nervous system of cattle with various congenital manifestations. Zentralbl Veterinarmed B. 1992;39:599–609. doi: 10.1111/j.1439-0450.1992.tb01211.x. [DOI] [PubMed] [Google Scholar]