Abstract
Background
Higher levels of physical activity are associated with fewer cardiovascular disease (CVD) events. While the precise mechanisms underlying this inverse association are unclear, differences in several cardiovascular risk factors may mediate this effect.
Methods and Results
In a prospective study of 27,055 apparently healthy women, we measured baseline levels of hemoglobin A1c, traditional lipids (total, LDL, and HDL cholesterol), novel lipids (lipoprotein [a], apolipoprotein A1 and B100), creatinine, homocysteine, inflammatory/hemostatic biomarkers (high-sensitivity C-reactive protein, fibrinogen, soluble intracellular adhesion molecule-1), and women self-reported physical activity, weight, height, hypertension and diabetes. Mean follow-up was 10.9±1.6 years, and 979 incident CVD events occurred. The risk of CVD decreased linearly with higher levels of activity (P, linear trend, <0.001). Using the reference group of <200 kcal/week of activity, the age and treatment-adjusted relative risk reductions associated with 200–599, 600–1499, and ≥1500 kcal/week were 27%, 32%, and 41%, respectively. Differences in known risk factors explained a large proportion (59.0%) of the observed inverse association. When sets of risk factors were examined, inflammatory/hemostatic biomarkers made the largest contribution to lower risk (32.6%), followed by blood pressure (27.1%). Novel lipids contributed less to CVD risk reduction, compared with traditional lipids (15.5% and 19.1%, respectively). Smaller contributions were attributed to body mass index (10.1%) and hemoglobin A1c/diabetes (8.9%), while homocysteine and creatinine had negligible effects (<1%).
Conclusions
The inverse association between physical activity and CVD risk is mediated in substantial part by known risk factors, particularly inflammatory/hemostatic factors and blood pressure.
Keywords: epidemiology, exercise, risk factors, cardiovascular diseases
Physical activity or fitness clearly reduces the risk of cardiovascular disease (CVD), with a magnitude of risk reduction comparable to that of not smoking.1,2 However, the precise mechanisms through which physical activity lowers CVD risk are not well understood. Even after accounting for traditional cardiovascular risk factors such as blood pressure, lipids and diabetes, the inverse relation between physical activity and CVD risk persists.3–7 Changes in individual risk factors with physical activity tend to be modest, on the order of 5% for blood lipids,8,9 3–5 mmHg for blood pressure,10,11 and 1% for hemoglobin A1c,12 in contrast to the large reductions (30–50%) in CVD risk seen with physical activity.
The relative contribution of these various risk factors, representing a number of physiological pathways, towards the activity-related risk reduction in CVD is unknown. In addition, newly-recognized CVD risk factors, in particular those relating to inflammation and hemostasis, are also modified favorably with physical activity.13–18 This likely represents an additional mechanistic pathway through which physical activity decreases CVD risk. Therefore, the aim of the present study was to quantify the contribution of traditional and novel risk factors to the activity-related reduction in CVD.
METHODS
Study Population
Study participants were drawn from the Women’s Health Study (WHS), a recently completed trial of low-dose aspirin and vitamin E in the primary prevention of CVD in women.19–21 WHS participants were apparently healthy female health care professionals, ages 45 years or older, who were free of self-reported CVD and cancer at study entry (1992–1995). Women gave written informed consent and completed questionnaires at the time of enrollment on demographics, anthropometrics, medical history, medications, and lifestyle factors. They were also asked to provide a blood sample; 28,345 women did so. For this study, we excluded women with missing data on physical activity or body mass index (N=544), or missing information on the traditional or novel biomarkers of interest (N=433), leaving 27,055 women for analysis. The study was approved by the institutional review board of the Brigham and Women’s Hospital (Boston, Mass).
Assessment of Physical Activity and CVD Risk Factors
Physical activity was assessed at study entry using a questionnaire that has been shown to be valid and reliable.22 The correlation of activity reported on the questionnaires, compared with activity diaries kept for 4 weeks over a year, was 0.62.22 Participants were asked to estimate the average time per week over the past year spent on 8 groups of recreational activities and the number of flights of stairs climbed daily.23 A metabolic equivalent task (MET) score was assigned to each activity based on its energy cost. We estimated the energy expended on each of the above groups of activities, and summed over all activities to estimate the total energy expended on physical activity (kcal/week). We used kilocalories as the unit of energy expenditure because this unit is widely understood by physicians and patients. However, body weight is used in the computation of energy expenditure in kcal/week (i.e., for the same activity, a heavier person expends more kilocalories than a lighter person). Thus, we repeated analyses using energy expenditure estimated in MET-hours, a unit that is independent of body weight (i.e., for the same activity, heavier and lighter persons expend the same MET-hours).
At study entry, participants also reported information on smoking, diet, menopausal status, hormone use, weight, height, blood pressure, history of hypertension (blood pressure ≥140/90 or anti-hypertensive medication use) and diabetes, and family history.
Laboratory Measurements
EDTA blood samples were obtained at the time of enrollment into the WHS and stored in vapor phase liquid nitrogen (−170° C). Hemoglobin A1c was assessed using an immunoturbidimetric assay (Roche Diagnostics, Indianapolis, IN). Total, LDL, and HDL cholesterol were assayed using reagents from Genzyme Corporation (Cambridge, Mass) and Roche Diagnostics. Lipoprotein (a) was measured using an immunoturbidimetric assay (Roche Diagnostics) with reagents and calibrators from Denka Seiken (Tokyo, Japan). Apolipoproteins A1 and B100 were measured using immunoturbidimetric assays (DiaSorin, Stillwater, Minn). Creatinine was measured by a rate-blanked method that is based on the Jaffé reaction using Roche Diagnostics reagents. An enzymatic assay was used to measure homocysteine (Catch Inc., Seattle, Wash). C-reactive protein (hsCRP) was measured using a high-sensitivity immunoturbidimetric assay on the Hitachi 917 analyzer (Roche Diagnostics), using reagents and calibrators from Denka Seiken. Fibrinogen was measured using an immunoturbidimetric assay (Kamiya Biomedical, Seattle, Wash) and soluble ICAM-1 was measured with an ELISA assay (R&D systems, Minneapolis, Minn).
Ascertainment of CVD Events
The primary endpoint of interest was a composite endpoint of incident CVD (nonfatal myocardial infarction [MI], nonfatal ischemic stroke, percutaneous coronary intervention [PCI], coronary artery bypass grafting [CABG], or cardiovascular death). Other endpoints were incident CHD (nonfatal MI, PCI, CABG, or coronary death), and the individual CVD endpoints. Women reported the endpoints of interest on follow-up questionnaires every 6 or 12 months, and confirmed events were included in analyses as previously described.21
Statistical Analysis
Statistical analyses were performed using STATA version 8.2 (STATA Corporation, College Station, Texas). We categorized participants into approximate quartiles of energy expenditure (<200, 200–599, 600–1499, and 1500 or more kcal/week), where the highest activity category corresponds to approximately 5 hours of moderate-intensity activity/week.23 Cox proportional hazard regression models were used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) according to these activity groups. Tests for linear trend were performed using the median value for each activity group. All P-values were two-tailed.
To examine the extent to which various CVD risk factors contributed to the risk reduction in events associated with activity, we initially considered each risk factor separately in a model that adjusted for age and randomized treatment assignment. We considered the magnitude of change in the HRs for the most active women, compared with the least, with and without adjustment for each risk factor. A larger change in the HR towards the null implies a larger mediating effect of that risk factor on the activity-related reduction in CVD.
Then, on an a priori basis, we grouped together a set of variables that are generally considered to be potential confounders rather than mediators (smoking, dietary intake of alcohol, fruits and vegetables, saturated fat, fiber, menopause, hormone use, and parental history of myocardial infarction <60 years old). We included this set of variables, together with age and randomized treatment assignment in a single model, referring to this model as the “basic model.”
Also on an a priori basis, we grouped other CVD risk factors, generally considered to be potential mediators, into sets of risk factors on the basis of their pathophysiological effects. Blood pressure and the presence or absence of hypertension were combined as one set. Hemoglobin A1c and the presence or absence of diabetes were combined as another set. To consider the combined effect of traditional lipids, we combined total, LDL, and HDL cholesterol into one set, with a similar analysis for novel lipids (lipoprotein [a], apolipoprotein A1 and B100). HsCRP, fibrinogen, and soluble ICAM-1 were considered as a group related to inflammatory and hemostatic pathways. Finally, body mass index and homocysteine were examined separately.
To examine the extent to which CVD risk factors potentially mediated the effect of activity on incident CVD, we next added these risk factors, one set at a time, to the basic model and examined the magnitude of change in the HRs for the most active women, compared with the least, without (basic model) and with adjustment for each set of risk factors (“adjusted model”). Finally, we performed a fully-adjusted analysis that included all the CVD risk factors simultaneously. The proportion of CVD risk reduction explained by each set of CVD risk factors was computed as:24,25 [(HRbasic model – HRadjusted model)/(HRbasic model -1)] X 100%.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
RESULTS
Table 1 shows the baseline characteristics of participants according to their activity levels. Active women had a healthier lifestyle, weighed less, and had better risk factor profiles than inactive women. There were modest, but statistically significant, differences in all biomarkers except for lipoprotein (a), with higher activity associated with better profiles (P for linear trend, <0.001).
TABLE 1.
Baseline Characteristics of Participants According to Physical Activity
| Physical Activity, kcal/week | ||||
|---|---|---|---|---|
| <200 N = 6789 | 200–599 N = 6732 | 600–1499 N = 7681 | ≥1500 N = 5853 | |
| Age, mean (SD), y | 54.7 (7.1) | 54.4 (7.0) | 54.8 (7.1) | 54.7 (7.1) |
| Current smoking, % | 17.1 | 11.9 | 9.1 | 7.9 |
| Alcohol consumption, % | ||||
| Rarely | 52.3 | 44.5 | 40.6 | 38.2 |
| 1–3 drinks/mo | 12.6 | 13.6 | 13.5 | 13.5 |
| 1–6 drinks/wk | 26.2 | 31.2 | 35.0 | 37.0 |
| ≥ 1 drink/d | 8.9 | 10.7 | 10.8 | 11.2 |
| Fruit and vegetable intake, mean (SD), servings/d | 5.2 (3.3) | 5.9 (3.2) | 6.4 (3.6) | 7.3 (4.0) |
| Saturated fat intake, mean (SD), g/d | 20.5 (8.5) | 20.2 (7.9) | 19.3 (7.7) | 18.8 (7.9) |
| Fiber intake, mean (SD), g/d | 16.7 (7.4) | 18.7 (7.6) | 19.8 (8.0) | 21.6 (9.2) |
| Hypertension, % | 28.6 | 24.7 | 23.0 | 23.8 |
| Diabetes, % | 3.5 | 2.5 | 2.5 | 2.4 |
| Postmenopausal status, % | 54.9 | 53.1 | 54.2 | 54.8 |
| Postmenopausal hormone use, % | 41.4 | 43.9 | 44.3 | 44.6 |
| Body mass index, mean (SD), kg/m2 | 26.9 (5.6) | 25.9 (4.9) | 25.3 (4.5) | 25.6 (4.7) |
| Parental history of myocardial infarction <60 years, % | 13.6 | 12.4 | 12.6 | 12.8 |
| Biomarkers, median (IQR) | ||||
| Hemoglobin A1c, mg/dL | 5.03 (4.86–5.23) | 5.00 (4.84–5.19) | 4.99 (4.83–5.18) | 4.99 (4.83–5.17) |
| Total cholesterol, mg/dL | 210 (186–237) | 209 (184–236) | 207 (183–234) | 207 (182–234) |
| LDL cholesterol, mg/dL | 124 (103–147) | 122 (102–145) | 120 (99–143) | 119 (98–142) |
| HDL cholesterol, mg/dL | 49.5 (41.3–60.2) | 51.6 (43.4–61.9) | 53.1 (44.1–63.1) | 53.5 (44.1–64.3) |
| Lipoprotein(a), mg/dL | 10.4 (4.3–31.0) | 10.9 (4.4–34.0) | 10.6 (4.6–32.6) | 10.5 (4.4–33.1) |
| Apolipoprotein A1, mg/dL | 146 (130–164) | 149 (133–167) | 150 (134–169) | 151 (134–170) |
| Apolipoprotein B100, mg/dL | 105 (87–124) | 103 (84–122) | 99 (83–119) | 98 (82–119) |
| Creatinine, mg/dL | 0.70 (0.62–0.79) | 0.71 (0.63–0.80) | 0.71 (0.63–0.80) | 0.72 (0.64–0.81) |
| Homocysteine, μmol/L | 10.8 (8.9–13.3) | 10.4 (8.6–12.8) | 10.3 (8.7–12.6) | 10.4 (8.6–12.8) |
| High-sensitivity C-reactive protein, mg/L | 2.5 (1.0–5.1) | 2.0 (0.8–4.4) | 1.8 (0.7–4.1) | 1.8 (0.7–3.8) |
| Fibrinogen, mg/dL | 362 (315–416) | 351 (308–402) | 347 (305–398) | 344 (303–395) |
| Soluble intracellular adhesion molecule-1, ng/mL | 356 (309–414) | 342 (301–394) | 338 (297–386) | 337 (298–384) |
P values across physical activity categories were all <0.05 except for postmenopausal status (P=0.05), parental history (P=0.20), and lipoprotein (a) (P=0.25).
During a mean follow-up of 10.9±1.6 years, a total of 979 first CVD events occurred, including 640 CHD events (253 MI, 398 PCI, and 219 CABG) and 266 ischemic strokes. The risk of incident CVD decreased linearly with higher levels of activity (P for linear trend, <0.001; Table 2). Using the reference group of <200 kcal/week of activity and after adjusting for age and randomized-treatment assignment, the relative risk reductions associated with 200–599, 600–1499, and ≥1500 kcal/week were 27%, 32%, and 41%, respectively. In separate Cox regression models which considered each risk factor variable, one at a time, and adjusted for age and treatment assignment (Table 2), there was some attenuation noted in the HRs comparing the most active women with the least active, before and after adjustment for all variables, except for creatinine, lipoprotein (a), and postmenopausal status/hormone use. However, when all risk factors were combined together in one model, the HR comparing the most active women with the least active was substantially attenuated (0.90, 95% CI 0.73–1.11, after adjusting for all risk factors, compared with 0.59, 95% CI 0.49–0.71, after only adjusting for age and treatment assignment), and the linear trend across activity levels was no longer significant (P for linear trend, 0.37).
TABLE 2.
Association of Physical Activity with Cardiovascular Disease Events after Adjusting for CVD Risk Factors
| Physical Activity, kcal/week | |||||
|---|---|---|---|---|---|
| <200 | 200–599 | 600–1499 | ≥1500 | P for Trend | |
| Hazard Ratios (95% Confidence Intervals) | |||||
| Age- and treatment-adjusted model | 1.00 | 0.73 (0.61–0.86) | 0.68 (0.57–0.80) | 0.59 (0.49–0.71) | <0.001 |
| Age- and treatment-adjusted plus each of the following added one at a time | |||||
| Smoking | 1.00 | 0.76 (0.64–0.90) | 0.72 (0.61–0.85) | 0.62 (0.52–0.75) | <0.001 |
| Alcohol consumption | 1.00 | 0.73 (0.62–0.87) | 0.69 (0.58–0.81) | 0.60 (0.50–0.73) | <0.001 |
| Fruit, vegetable, saturated fat, fiber intake | 1.00 | 0.76 (0.64–0.90) | 0.71 (0.60–0.84) | 0.66 (0.54–0.80) | <0.001 |
| Postmenopausal status, hormone use | 1.00 | 0.74 (0.62–0.87) | 0.68 (0.58–0.81) | 0.59 (0.49–0.72) | <0.001 |
| Parental history of MI <60 years | 1.00 | 0.77 (0.64–0.92) | 0.71 (0.60–0.85) | 0.62 (0.51–0.76) | <0.001 |
| Hypertension | 1.00 | 0.75 (0.64–0.89) | 0.71 (0.60–0.84) | 0.62 (0.51–0.74) | <0.001 |
| Blood pressure | 1.00 | 0.77 (0.65–0.92) | 0.75 (0.63–0.88) | 0.66 (0.54–0.79) | <0.001 |
| Diabetes | 1.00 | 0.75 (0.64–0.89) | 0.69 (0.59–0.82) | 0.61 (0.51–0.74) | <0.001 |
| Hemoglobin A1c | 1.00 | 0.76 (0.64–0.90) | 0.72 (0.61–0.85) | 0.63 (0.52–0.76) | <0.001 |
| Body mass index | 1.00 | 0.77 (0.65–0.91) | 0.74 (0.63–0.87) | 0.63 (0.52–0.76) | <0.001 |
| Total cholesterol | 1.00 | 0.73 (0.62–0.87) | 0.69 (0.58–0.81) | 0.60 (0.50–0.72) | <0.001 |
| LDL cholesterol | 1.00 | 0.73 (0.62–0.87) | 0.69 (0.58–0.81) | 0.60 (0.50–0.72) | <0.001 |
| HDL cholesterol | 1.00 | 0.76 (0.64–0.90) | 0.72 (0.61–0.85) | 0.64 (0.53–0.77) | <0.001 |
| Lipoprotein (a) | 1.00 | 0.72 (0.61–0.85) | 0.67 (0.57–0.79) | 0.58 (0.48–0.70) | <0.001 |
| Apolipoprotein A1 | 1.00 | 0.75 (0.63–0.89) | 0.70 (0.60–0.83) | 0.62 (0.51–0.74) | <0.001 |
| Apolipoprotein B100 | 1.00 | 0.75 (0.63–0.89) | 0.72 (0.61–0.84) | 0.62 (0.52–0.75) | <0.001 |
| Creatinine | 1.00 | 0.73 (0.61–0.86) | 0.67 (0.57–0.80) | 0.59 (0.49–0.71) | <0.001 |
| Homocysteine | 1.00 | 0.73 (0.62–0.87) | 0.69 (0.58–0.81) | 0.60 (0.49–0.72) | <0.001 |
| High-sensitivity C-reactive protein | 1.00 | 0.77 (0.65–0.91) | 0.73 (0.62–0.86) | 0.64 (0.53–0.77) | <0.001 |
| Fibrinogen | 1.00 | 0.76 (0.64–0.89) | 0.72 (0.61–0.85) | 0.63 (0.52–0.76) | <0.001 |
| Soluble intracellular adhesion molecule-1 | 1.00 | 0.77 (0.65–0.91) | 0.73 (0.62–0.86) | 0.64 (0.53–0.77) | <0.001 |
| All the above in one model | 1.00 | 0.95 (0.79–1.15) | 0.95 (0.79–1.14) | 0.90 (0.73–1.11) | 0.37 |
Next, to determine the extent to which the reduced risk of CVD associated with activity was influenced by potential mediators representing various physiological pathways, each set of mediators was added, one set at a time, to the basic model (Table 3, top panel). For CVD, the addition of blood pressure/hypertension resulted in an attenuation of the inverse relation, which became non-significant (P, trend=0.09), with similar results for the inflammatory/hemostatic biomarkers (P, trend=0.10). The addition of body mass index, hemoglobin A1c/diabetes, traditional lipids, and novel lipids, one set at a time, resulted in smaller attenuations in the inverse relation between activity and CVD (all P, trend ≤0.05). When all sets of risk factors were added simultaneously to the basic model, this resulted in further attenuation of the HRs, and no significant associations were observed (P, trend=0.36).
TABLE 3.
Association of Physical Activity with Cardiovascular and Coronary Heart Disease Events after Adjusting for Sets of Potential Mediators
| Physical Activity, kcal/week | |||||
|---|---|---|---|---|---|
| <200 | 200–599 | 600–1499 | ≥1500 | P for Trend | |
| Hazard Ratios (95% Confidence Intervals) Cardiovascular Disease | |||||
| Age- and treatment-adjusted model | 1.00 | 0.73 (0.61–0.86) | 0.68 (0.57–0.80) | 0.59 (0.49–0.71) | <0.001 |
| Basic model* | 1.00 | 0.86 (0.72–1.04) | 0.82 (0.68–0.98) | 0.75 (0.61–0.93) | 0.01 |
| Basic model plus each set of risk factors below, added one group at a time:† | |||||
| Blood pressure/hypertension | 1.00 | 0.91 (0.75–1.09) | 0.89 (0.74–1.08) | 0.82 (0.66–1.01) | 0.09 |
| Body mass index | 1.00 | 0.90 (0.75–1.08) | 0.88 (0.73–1.05) | 0.78 (0.63–0.96) | 0.02 |
| Hemoglobin A1c/diabetes | 1.00 | 0.89 (0.74–1.07) | 0.83 (0.69–1.00) | 0.77 (0.62–0.95) | 0.02 |
| Traditional lipids Total, LDL, HDL cholesterol | 1.00 | 0.90 (0.74–1.08) | 0.87 (0.72–1.05) | 0.80 (0.65–0.99) | 0.05 |
| Novel lipids Lp(a), Apo A1, Apo B100 | 1.00 | 0.88 (0.73–1.06) | 0.85 (0.71–1.02) | 0.79 (0.64–0.97) | 0.04 |
| Homocysteine | 1.00 | 0.87 (0.72–1.04) | 0.82 (0.68–0.99) | 0.75 (0.61–0.93) | 0.01 |
| Inflammatory/hemostatic hsCRP, fibrinogen, sICAM-1 | 1.00 | 0.93 (0.77–1.12) | 0.91 (0.75–1.09) | 0.83 (0.67–1.03) | 0.10 |
| All of the above†† | 1.00 | 0.95 (0.79–1.15) | 0.95 (0.79–1.14) | 0.90 (0.73–1.11) | 0.36 |
| Coronary Heart Disease | |||||
| Age- and treatment-adjusted model | 1.00 | 0.71 (0.58–0.87) | 0.64 (0.52–0.78) | 0.48 (0.38–0.62) | <0.001 |
| Basic model | 1.00 | 0.84 (0.67––1.06) | 0.76 (0.61–0.96) | 0.62 (0.48–0.82) | 0.001 |
| Basic model plus each set of risk factors below, added one group at a time: | |||||
| Blood pressure/hypertension | 1.00 | 0.88 (0.70–1.11) | 0.84 (0.67–1.05) | 0.68 (0.52–0.89) | 0.006 |
| Body mass index | 1.00 | 0.89 (0.71–1.11) | 0.83 (0.66–1.04) | 0.65 (0.50–0.85) | 0.002 |
| Hemoglobin A1c/diabetes | 1.00 | 0.87 (0.69–1.08) | 0.78 (0.62–0.98) | 0.64 (0.49–0.84) | 0.001 |
| Traditional lipids Total, LDL, HDL cholesterol | 1.00 | 0.88 (0.70–1.10) | 0.83 (0.66–1.04) | 0.67 (0.52–0.88) | 0.005 |
| Novel lipids Lp(a), Apo A1, Apo B100 | 1.00 | 0.86 (0.69–1.08) | 0.81 (0.64–1.01) | 0.67 (0.51–0.87) | 0.004 |
| Homocysteine | 1.00 | 0.85 (0.68–1.06) | 0.77 (0.61–0.96) | 0.63 (0.48–0.82) | 0.001 |
| Inflammatory/hemostatic hsCRP, fibrinogen, sICAM-1 | 1.00 | 0.91 (0.73–1.14) | 0.86 (0.68–1.08) | 0.70 (0.54–0.92) | 0.01 |
| All of the above | 1.00 | 0.93 (0.74–1.17) | 0.89 (0.71–1.13) | 0.76 (0.58–0.99) | 0.05 |
Basic models included age, randomized treatment assignment, smoking, consumption of alcohol, fruits and vegetables, saturated fat, fiber, menopausal status, postmenopausal hormone use, and parental history of myocardial infarction.
Models were adjusted for the variables in the basic model plus each of the sets of risk factors added one group at a time, to separate models.
Model included variables in the basic model, plus all sets of risk factors included simultaneously in one model.
For CHD, a broadly similar pattern was observed (Table 3, bottom panel). Unlike CVD, there remained a borderline significant inverse association with CHD, after adjustment for all sets of risk factors (P, trend=0.05).
The associations of activity with nonfatal MI, PCI, and CABG, when examined as separate endpoints, are shown in Table 4. When the sets of potential mediators were added to the basic model, the effect of physical activity was attenuated in a manner similar to that seen with CHD. The association of activity with ischemic stroke was non-linear (corresponding age and treatment-adjusted HRs and 95% CIs: 1.00, 0.68 [0.49–0.95], 0.69 [0.50–0.95], and 0.72 [0.51–1.01], respectively; P, trend=0.16).
TABLE 4.
Association of Physical Activity with Myocardial Infarction and Coronary Revascularization Procedures after Adjusting for Sets of Potential Mediators
| Physical Activity, kcal/week | |||||
|---|---|---|---|---|---|
| <200 | 200–599 | 600–1499 | ≥1500 | P for Trend | |
| Hazard Ratios (95% Confidence Intervals) | |||||
| Myocardial Infarction | |||||
| Age- and treatment-adjusted model | 1.00 | 0.63 (0.45–0.87) | 0.62 (0.45–0.85) | 0.44 (0.30–0.65) | <0.001 |
| Basic model | 1.00 | 0.77 (0.54–1.11) | 0.79 (0.56–1.13) | 0.53 (0.34–0.83) | 0.009 |
| Basic model plus each set of risk factors below, added one group at a time: | |||||
| Blood pressure/hypertension | 1.00 | 0.84 (0.58–1.20) | 0.89 (0.62–1.28) | 0.58 (0.37–0.91) | 0.03 |
| Body mass index | 1.00 | 0.81 (0.56–1.16) | 0.85 (0.60–1.22) | 0.55 (0.35–0.86) | 0.01 |
| Hemoglobin A1c/diabetes | 1.00 | 0.81 (0.56–1.16) | 0.82 (0.57–1.17) | 0.55 (0.35–0.86) | 0.01 |
| Traditional lipids Total, LDL, HDL cholesterol | 1.00 | 0.81 (0.56–1.16) | 0.86 (0.60–1.22) | 0.57 (0.36–0.89) | 0.02 |
| Novel lipids Lp(a), Apo A1, Apo B100 | 1.00 | 0.79 (0.55–1.13) | 0.83 (0.58–1.18) | 0.56 (0.36–0.87) | 0.02 |
| Homocysteine | 1.00 | 0.78 (0.54–1.12) | 0.80 (0.56–1.14) | 0.53 (0.34–0.83) | 0.01 |
| Inflammatory/hemostatic hsCRP, fibrinogen, sICAM-1 | 1.00 | 0.83 (0.57–1.19) | 0.87 (0.61–1.25) | 0.58 (0.37–0.91) | 0.03 |
| All of the above | 1.00 | 0.88 (0.61–1.28) | 0.95 (0.66–1.36) | 0.64 (0.41–1.00) | 0.07 |
| Percutaneous Coronary Intervention | |||||
| Age- and treatment-adjusted model | 1.00 | 0.74 (0.57–0.95) | 0.55 (0.42–0.71) | 0.44 (0.33–0.60) | <0.001 |
| Basic model | 1.00 | 0.90 (0.68–1.18) | 0.67 (0.50–0.90) | 0.58 (0.41–0.83) | 0.001 |
| Basic model plus each set of risk factors below, added one group at a time: | |||||
| Blood pressure/hypertension | 1.00 | 0.94 (0.71–1.24) | 0.72 (0.54–0.97) | 0.63 (0.44–0.89) | 0.004 |
| Body mass index | 1.00 | 0.95 (0.72–1.25) | 0.73 (0.54–0.99) | 0.61 (0.43–0.87) | 0.002 |
| Hemoglobin A1c/diabetes | 1.00 | 0.92 (0.70–1.21) | 0.69 (0.51–0.93) | 0.60 (0.42–0.86) | 0.002 |
| Traditional lipids Total, LDL, HDL cholesterol | 1.00 | 0.95 (0.72–1.25) | 0.74 (0.55–1.00) | 0.64 (0.45–0.91) | 0.006 |
| Novel lipids Lp(a), Apo A1, Apo B100 | 1.00 | 0.93 (0.70–1.22) | 0.71 (0.53–0.96) | 0.63 (0.45–0.90) | 0.005 |
| Homocysteine | 1.00 | 0.90 (0.68–1.18) | 0.67 (0.50–0.90) | 0.58 (0.41–0.83) | 0.001 |
| nflammatory/hemostatic hsCRP, fibrinogen, sICAM-1 | 1.00 | 0.97 (0.74–1.28) | 0.76 (0.56–1.02) | 0.66 (0.46–0.94) | 0.009 |
| All of the above | 1.00 | 1.01 (0.76–1.33) | 0.79 (0.59–1.07) | 0.71 (0.50–1.01) | 0.03 |
| Coronary Artery Bypass Graft Surgery | |||||
| Age- and treatment-adjusted model | 1.00 | 0.64 (0.44–0.92) | 0.74 (0.53–1.04) | 0.64 (0.43–0.94) | 0.09 |
| Basic model | 1.00 | 0.70 (0.46–1.05) | 0.82 (0.56–1.21) | 0.80 (0.52–1.22) | 0.58 |
| Basic model plus each set of risk factors below, added one group at a time: | |||||
| Blood pressure/hypertension | 1.00 | 0.72 (0.48–1.10) | 0.94 (0.64–1.39) | 0.89 (0.58–1.38) | 1.00 |
| Body mass index | 1.00 | 0.74 (0.49–1.11) | 0.90 (0.61–1.32) | 0.83 (0.54–1.28) | 0.69 |
| Hemoglobin A1c/diabetes | 1.00 | 0.73 (0.48–1.10) | 0.85 (0.58–1.25) | 0.85 (0.55–1.31) | 0.74 |
| Traditional lipids Total, LDL, HDL cholesterol | 1.00 | 0.73 (0.49–1.10) | 0.90 (0.61–1.32) | 0.87 (0.56–1.33) | 0.84 |
| Novel lipids Lp(a), Apo A1, Apo B100 | 1.00 | 0.71 (0.47–1.08) | 0.88 (0.60–1.29) | 0.84 (0.55–1.30) | 0.77 |
| Homocysteine | 1.00 | 0.70 (0.46–1.05) | 0.83 (0.56–1.21) | 0.80 (0.52–1.23) | 0.58 |
| Inflammatory/hemostatic hsCRP, fibrinogen, sICAM-1 | 1.00 | 0.76 (0.51–1.15) | 0.94 (0.64–1.38) | 0.92 (0.60–1.41) | 1.00 |
| All of the above | 1.00 | 0.78 (0.51–1.19) | 1.04 (0.70–1.53) | 1.04 (0.67–1.61) | 0.54 |
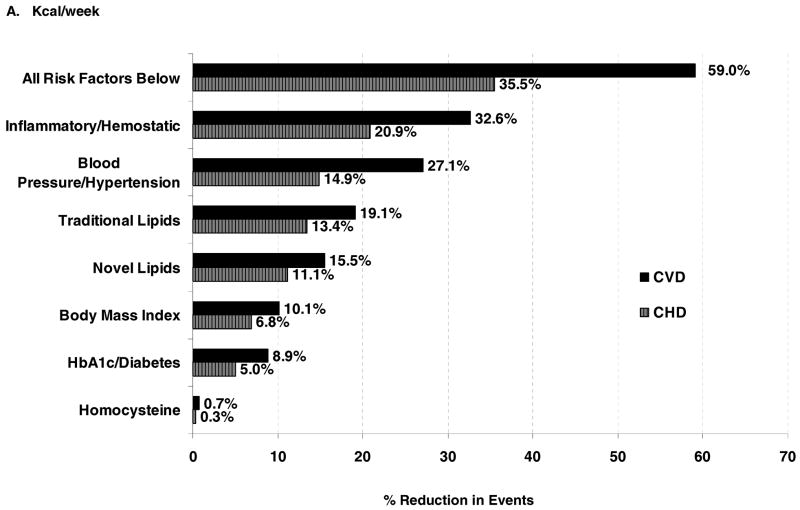
Finally, we computed the proportion of the physical activity-related reduction in CVD or CHD events explained by each set of potential mediators (Figure). A large proportion (59.0%) of the inverse relation between physical activity and CVD risk was explained by the potential mediators that we investigated. When examined as sets of risk factors, inflammatory/hemostatic biomarkers were the largest contributors to lower risk (32.6%), followed by blood pressure (27.1%). Novel lipids (lipoprotein [a], apolipoprotein A1 and B100) contributed less to CVD risk reduction compared with traditional lipids (total, LDL, and HDL cholesterol); 15.5% and 19.1%, respectively. Smaller contributions were attributed to body mass index (10.1%) and hemoglobin A1c/diabetes (8.9%), while homocysteine had negligible effects (<1%). A similar pattern, but smaller was observed for CHD (35.5% of the CHD event risk reduction explained by risk factors, compared with 59.0% for CVD).
Figure.
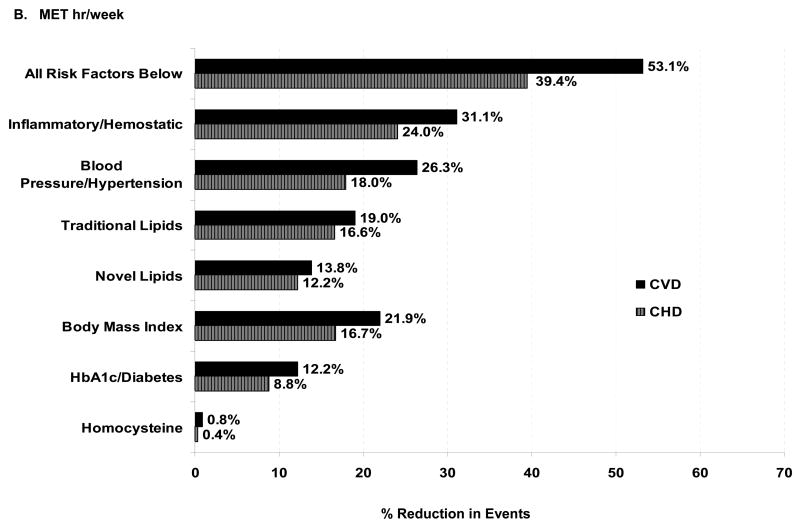
Percentage reduction in CVD events associated with physical activity that is explained by risk factors. The proportion of the risk reduction for ≥1500 kcal/wk of physical activity (compared with the reference group of <200 kcal/wk, Panel A), and for >20.5 MET hrs/wk (compared with the reference group of <2.8 MET hrs/wk, Panel B), that is explained by each set of potential risk factors, calculated as (HRbasic model – HRadjusted model)/(HRbasic model -1) X 100%.24 These proportions were calculated from HRs expressed up to 5 decimal points for greater accuracy, and thus may differ slightly from the data shown in Table 3.
We repeated our analyses (Figure, Panel B), calculating activity expenditure in MET-hours/week instead of kcal/week. Almost identical results were obtained, except for body mass index, whose contribution increased from 10.1% to 21.9%, and hemoglobin A1c/diabetes, which increased from 8.9% to 12.2%.
DISCUSSION
While physical activity has clearly been shown to reduce the risk of developing CVD, the biological mechanisms underlying this association are unclear, as is the extent to which various pathways might underlie the inverse association. This study indicates that the association between higher levels of activity and lower CVD rates can be explained in large part by known risk factors, both traditional and novel. The risk factors that were investigated in this study explained 59.0% of the activity-related reduction in CVD, with inflammatory/hemostatic biomarkers making the largest contribution to lowered risk, followed by blood pressure, lipids, and body mass index. A smaller contribution was attributed to measures of glucose abnormalities, with minimal contribution observed from measures of renal function or homocysteine.
The beneficial effect of physical activity was stronger for CHD compared with CVD in this study, but the relative contributions of potential mediators were proportional and qualitatively similar. Previous studies have noted non-linear associations, weak or positive associations between activity and stroke,26,27 as did we.
Prior studies have demonstrated favorable effects of physical activity on traditional risk factors.9,28–30 While some individuals may experience large changes in risk factors with exercise,31 most individuals experience modest short-term changes, on the order of 2–5%.8–12 The effect of exercise on inflammatory factors has been recognized more recently.13,17,32 Acute bouts of exercise result in a transient, mostly pro-inflammatory, several-fold increase in acute-phase reactants and cytokines,33 proportional to the amount of exercise and muscle injury.32
By contrast, regular activity has been associated also with a chronic anti-inflammatory effect, with moderate (~20–30%) reductions in CRP and soluble intracellular and vascular adhesion molecules.34,35 We previously demonstrated that in this population of women, the highest vs lowest level of activity was associated with approximately 43% lower hsCRP level, which was mildly attenuated to 37% after adjusting for the other risk factors, including body mass index.18,36 The mechanisms underlying the chronic anti-inflammatory and hemostatic effects of exercise are not well defined and are only partially related to body weight.18,36 Other explanations include possible effects on proatherogenic adipokines, insulin-sensitizing pathways, or the hemostatic and antioxidant functions of the coronary endothelium.32,37 Regular exercise attenuates the age-associated increase in oxidative stress and nuclear factor kappaB activation in animals,38 and reduces toll-like receptor 4 (TLR4) signaling which may explain the chronic anti-inflammatory effect of exercise.39–40
Several limitations of the present study warrant consideration. Physical activity and several of the risk factors were assessed by self-report. It is possible that more precise assessment of these factors may have resulted in a different contribution of these variables to the reduction in CVD risk. The study design was observational but prospectively collected. We did not have information on other variables that are favorably influenced by physical activity, such as those related to heart rate or autonomic balance,41 baseline waist circumference or insulin sensitivity,42 and nitric oxide-dependent endothelial activity,43 and so could not evaluate their contributions to the inverse relation between physical activity and CVD risk.
There are also several strengths of the study, including the large number of women investigated, detailed information on physical activity, a wide range of traditional and novel CVD risk factors, and the long duration of follow-up for the various cardiovascular endpoints.
In summary, to our knowledge, this is the first study attempting to quantify the relative importance of potential underlying mechanisms through which higher levels of physical activity are associated with lower risk of CVD events. In this study, we have identified potential underlying mechanisms through which even moderate levels of physical activity (at least 600 kcal/week, or the equivalent of just over 2 hours per week of brisk walking, consistent with current guideline recommendations)44 are associated with lower risk of clinically important CVD events. Modest changes in known CVD risk factors, particularly those relating to inflammation/hemostasis and blood pressure, account for a substantial portion of the benefit of physical activity on CVD risk, and thus may have important downstream consequences for the primary prevention of CVD.
Acknowledgments
Funding Sources
The research for this article was supported by grants from the Donald W. Reynolds Foundation, Leducq Foundation, and Doris Duke Charitable Foundation. The Women’s Health Study is supported by grants HL-43851 and CA-47988 from the National Heart, Lung, and Blood Institute, the National Cancer Institute, and philanthropic support from Elisabeth and Alan Doft and their family. Dr Mora is supported by the Sandra Daugherty Foundation and grant 0670007N from the American Heart Association. The funding agencies played no role in the design, conduct, data management, analysis, or manuscript preparation related to this manuscript.
Footnotes
Conflict of Interest Disclosures
Dr. Ridker is listed as a co-inventor on patents held by the Brigham and Women’s Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease. Dr. Lee has served as a consultant for Virgin Life Care and sits on their Scientific Advisory Board. The remaining authors report no conflicts.
References
- 1.Blair SN, Kampert JB, Kohl HW, 3rd, Barlow CE, Macera CA, Paffenbarger RS, Jr, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]
- 2.Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 3.Paffenbarger RS, Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314:605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 4.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 5.Bijnen FC, Caspersen CJ, Feskens EJ, Saris WH, Mosterd WL, Kromhout D. Physical activity and 10-year mortality from cardiovascular diseases and all causes: The Zutphen Elderly Study. Arch Intern Med. 1998;158:1499–1505. doi: 10.1001/archinte.158.14.1499. [DOI] [PubMed] [Google Scholar]
- 6.Stevens J, Cai J, Evenson KR, Thomas R. Fitness and fatness as predictors of mortality from all causes and from cardiovascular disease in men and women in the lipid research clinics study. Am J Epidemiol. 2002;156:832–841. doi: 10.1093/aje/kwf114. [DOI] [PubMed] [Google Scholar]
- 7.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 8.Leon AS, Sanchez OA. Response of blood lipids to exercise training alone or combined with dietary intervention. Med Sci Sports Exerc. 2001;33:S502–S515. doi: 10.1097/00005768-200106001-00021. [DOI] [PubMed] [Google Scholar]
- 9.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 10.Fagard RH. Exercise characteristics and the blood pressure response to dynamic physical training. Med Sci Sports Exerc. 2001;33:S484–S492. doi: 10.1097/00005768-200106001-00018. [DOI] [PubMed] [Google Scholar]
- 11.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 12.Thompson PD, Crouse SF, Goodpaster B, Kelley D, Moyna N, Pescatello L. The acute versus the chronic response to exercise. Med Sci Sports Exerc. 2001;33:S438–S445. doi: 10.1097/00005768-200106001-00012. [DOI] [PubMed] [Google Scholar]
- 13.Wannamethee SG, Lowe GD, Whincup PH, Rumley A, Walker M, Lennon L. Physical activity and hemostatic and inflammatory variables in elderly men. Circulation. 2002;105:1785–1790. doi: 10.1161/hc1502.107117. [DOI] [PubMed] [Google Scholar]
- 14.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. 2002;162:1286–1292. doi: 10.1001/archinte.162.11.1286. [DOI] [PubMed] [Google Scholar]
- 15.Eckel RH, Barouch WW, Ershow AG. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on the pathophysiology of obesity-associated cardiovascular disease. Circulation. 2002;105:2923–2928. doi: 10.1161/01.cir.0000017823.53114.4c. [DOI] [PubMed] [Google Scholar]
- 16.Church TS, Finley CE, Earnest CP, Kampert JB, Gibbons LW, Blair SN. Relative associations of fitness and fatness to fibrinogen, white blood cell count, uric acid and metabolic syndrome. Int J Obes Relat Metab Disord. 2002;26:805–813. doi: 10.1038/sj.ijo.0802001. [DOI] [PubMed] [Google Scholar]
- 17.Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL, Blair SN. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol. 2002;22:1869–1876. doi: 10.1161/01.atv.0000036611.77940.f8. [DOI] [PubMed] [Google Scholar]
- 18.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295:1412–1419. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 19.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 20.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 22.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 23.Lee IM, Rexrode KM, Cook NR, Manson JE, Buring JE. Physical activity and coronary heart disease in women: is “no pain, no gain” passe? JAMA. 2001;285:1447–1454. doi: 10.1001/jama.285.11.1447. [DOI] [PubMed] [Google Scholar]
- 24.Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia, Pa: Lippincott Williams & Wilkins; 1998. Measures of effect and association; pp. 47–64. [Google Scholar]
- 25.Shishehbor MH, Litaker D, Pothier CE, Lauer MS. Association of socioeconomic status with functional capacity, heart rate recovery, and all-cause mortality. JAMA. 2006;295:784–792. doi: 10.1001/jama.295.7.784. [DOI] [PubMed] [Google Scholar]
- 26.Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke. 2003;34:2475–2481. doi: 10.1161/01.STR.0000091843.02517.9D. [DOI] [PubMed] [Google Scholar]
- 27.Wendel-Vos GC, Schuit AJ, Feskens EJ, Boshuizen HC, Verschuren WM, Saris WH, Kromhout D. Physical activity and stroke. A meta-analysis of observational data. Int J Epidemiol. 2004;33:787–798. doi: 10.1093/ije/dyh168. [DOI] [PubMed] [Google Scholar]
- 28.Ivy JL. Role of insulin during exercise-induced glycogenesis in muscle: effect on cyclic AMP. Am J Physiol. 1977;233:E509–E513. doi: 10.1152/ajpendo.1977.233.6.E509. [DOI] [PubMed] [Google Scholar]
- 29.Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl HW, Blair SN. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness: a randomized trial. JAMA. 1999;281:327–334. doi: 10.1001/jama.281.4.327. [DOI] [PubMed] [Google Scholar]
- 30.Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. JAMA. 2003;290:1323–1330. doi: 10.1001/jama.290.10.1323. [DOI] [PubMed] [Google Scholar]
- 31.Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease. Circulation. 2003;107:3109–116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 32.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45:1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 33.Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK. A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol. 1998;513:889–894. doi: 10.1111/j.1469-7793.1998.889ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tisi PV, Hulse M, Chulakadabba A, Gosling P, Shearman CP. Exercise training for intermittent claudication: does it adversely affect biochemical markers of the exercise-induced inflammatory response? Eur J Vasc Endovasc Surg. 1997;14:344–350. doi: 10.1016/s1078-5884(97)80283-3. [DOI] [PubMed] [Google Scholar]
- 35.Adamopoulos S, Parissis J, Kroupis C, Georgiadis M, Karatzas D, Karavolias G, Koniavitou K, Coats AJ, Kremastinos DT. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur Heart J. 2001;22:791–797. doi: 10.1053/euhj.2000.2285. [DOI] [PubMed] [Google Scholar]
- 36.Hamer M. The relative influences of fitness and fatness on inflammatory factors. Prev Med. 2006;44:3–11. doi: 10.1016/j.ypmed.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 38.Radak Z, Chung HY, Naito H, Takahashi R, Jung KJ, Kim HJ, Goto S. Age-associated increase in oxidative stress and nuclear factor kappaB activation are attenuated in rat liver by regular exercise. FASEB J. 2004;18:749–750. doi: 10.1096/fj.03-0509fje. [DOI] [PubMed] [Google Scholar]
- 39.McFarlin BK, Flynn MG, Phillips MD, Stewart LK, Timmerman KL. Chronic resistance exercise training improves natural killer cell activity in older women. J Gerontol A Biol Sci Med Sci. 2005;60:1315–1318. doi: 10.1093/gerona/60.10.1315. [DOI] [PubMed] [Google Scholar]
- 40.Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, McFarlin BK, Timmerman KL, Coen PM, Felker J, Talbert E. Influence of exercise training and age on CD14+ cell-surface expression of toll-like receptor 2 and 4. Brain Behav Immun. 2005;19:389–397. doi: 10.1016/j.bbi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Lauer MS, Froelicher V. Abnormal heart-rate recovery after exercise. Lancet. 2002;360:1176–1177. doi: 10.1016/S0140-6736(02)11224-4. [DOI] [PubMed] [Google Scholar]
- 42.Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001;86:3257–3265. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 43.Zhao G, Zhang X, Xu X, Ochoa M, Hintze TH. Short-term exercise training enhances reflex cholinergic nitric oxide-dependent coronary vasodilation in conscious dogs. Circ Res. 1997;80:868–876. doi: 10.1161/01.res.80.6.868. [DOI] [PubMed] [Google Scholar]
- 44.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical Activity and Public Health: Updated Recommendation for Adults From the American College of Sports Medicine and the American Heart Association. Circulation. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]