Abstract
Background
Patients with primary progressive multiple sclerosis (PPMS) often develop severe disability despite low levels of abnormality on conventional magnetic resonance imaging (MRI). This may relate to diffuse pathological processes occurring in normal appearing brain tissue (NABT) involving both white matter (NAWM) and grey matter (NAGM). Magnetisation transfer imaging (MTI) is capable of identifying these processes and may be particularly informative when applied to patients with early PPMS.
Aim
To assess the relationship between abnormalities in NABT identified by MTI and disability and other radiological data in patients with early PPMS.
Methods
We studied 43 patients within 5 years of disease onset and 43 controls. The Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) were scored. Magnetisation transfer ratios (MTR) of NABT, NAWM, and NAGM were calculated and the following MTR parameters were measured: mean, peak height, peak location, and MTR value at the 25th, 50th, and 75th percentiles. Proton density, T2, T1, and gadolinium enhancing lesion loads were also calculated.
Results
Differences were found between patients and controls in mean, peak height, and peak location of NAWM and NAGM (p⩽0.001). Weak to moderate correlations were found between MTR parameters and disability in both NAWM and NAGM. Strong correlations between MTR parameters and lesion loads were found, particularly in NAWM.
Conclusion
MTR abnormalities are seen in NAWM and NAGM in early PPMS and both are associated with disability. NAWM MTR abnormalities are more closely related to conventional MRI measures than those seen in NAGM.
Keywords: early stage, magnetisation transfer imaging, normal appearing grey matter, normal appearing white matter, primary progressive multiple sclerosis
The mechanisms that underlie the development of disability in multiple sclerosis (MS) are not fully understood and there is evidence from magnetic resonance imaging (MRI) studies that there may be important pathological differences between primary progressive multiple sclerosis (PPMS) and the relapsing‐remitting form of the disease (RRMS).1 In PPMS, as in other forms of MS, it is likely that processes occurring within tissues that appear normal using conventional MRI techniques, contribute to the development of disability. Pathological changes in normal appearing white matter (NAWM) have previously been described in MS, including gliosis, inflammatory cell infiltration,2 and axonal loss.3 There is less information about pathological changes in normal appearing grey matter (NAGM), but cortical lesions have been demonstrated histologically4 and spectroscopic studies have reported reduced grey matter (GM) N‐acetyl‐aspartate.5 In PPMS, abnormalities within normal appearing brain tissues (NABT) have already been shown with newer MRI techniques,6 and have been found to be related to disability. However, it is not known how early these abnormalities arise in the disease course, as studies have tended to examine patients with well established disease. In addition, the relative contribution of abnormalities in white matter (WM) and GM has received little attention.
Conventional MRI mainly reflects the quantity of free water protons in tissue and is unable to detect protons bound to macromolecular structures due to their short T2 relaxation times. Magnetisation transfer imaging (MTI) makes use of the continuous magnetisation interchange between free protons and protons bound to macromolecules and provides an indirect measurement of tissue integrity. Magnetisation transfer ratio (MTR) is a quantitative measurement of the amount of magnetisation transfer occurring between these two pools of protons. A low MTR indicates a reduced capacity of the macromolecules in brain tissue to exchange magnetisation with the surrounding water molecules, and indicates structural tissue damage.7,8 The main pathological changes leading to reductions in MTR in MS are thought to be demyelination and axonal loss,9,10,11,12,13 with other pathological changes such as oedema, ischaemia, and gliosis having a lesser role.14 Post mortem studies have also found a correlation between low MTR and the percentage of remaining axons in MS lesions.15 Thus, this technique seems to have a greater pathological specificity than conventional MRI and may provide additional information on the widespread pathological processes which may be fundamental to the development of disability. MTI studies in MS have focussed largely on RRMS, secondary progressive MS, and clinically isolated syndromes. These studies showed reduced MTR in lesions,12,16 whole brain (WB),17 NAWM,12,18,19,20 and NAGM.19,21 A small number of studies have used MTI to examine patients with well established PPMS and have reported reductions of MTR in WB,22 NAWM,23 and NAGM.21 In these studies, the associations found with disability have been weak. This might be due to the inclusion of patients with very long disease duration.
The principal aim of this study is to determine whether MTR abnormalities in NAWM and NAGM are present in early PPMS and whether these relate to disability at this stage of the disease. A secondary aim is to study the relationship of MTR to conventional MR measurements in early PPMS.
Methods
Subjects
Forty three patients with PPMS fulfilling the diagnostic criteria for definite or probable PPMS according to Thompson et al,24 and who were within 5 years of clinical onset, were recruited from clinics at the National Hospital for Neurology and Neurosurgery and other hospitals in south east England. Patients were invited to take part in a 3 year longitudinal multimodal MRI study. Patients were subdivided into cord and non‐cord onset groups according to their clinical presentation. Forty three healthy controls were also studied.
All patients underwent conventional neurological assessment and were scored on Kurtzke's Expanded Disability Status Scale (EDSS)25 and the Multiple Sclerosis Functional Composite (MSFC).26 Using our own sample as a reference, z scores for the MSFC subtests (Paced Serial Auditory Test (z‐PASAT), Nine Hole Peg Test (z‐9HPT), and Timed Walk Test (z‐TWT)) were calculated according to published methods. The study was approved by the Joint Medical Ethics Committee of the National Hospital for Neurology and Neurosurgery and Institute of Neurology, London, UK. Written informed consent was obtained from all subjects before they were included in the study.
MRI acquisition
MRI of all subjects was carried out using a 1.5 T GE Signa scanner (General Electric, Milwaukee, IL, USA). In all subjects, WB MTI was acquired using a 2D interleaved spin echo (SE) sequence27 (28 contiguous 5 mm axial slices, repetition time (TR) 1720 ms, echo time (TE) 30/80 ms, number of excitations (NEX) 0.75, acquired matrix 256×128, reconstructed matrix 256×256, field of view (FOV) 240×240 mm) with and without a saturating Hamming‐apodised three lobe sinc MT pulse (duration of 16 ms, peak amplitude of 23.2 μT, bandwidth of 250 Hz applied 1 kHz off water resonance). Saturated and unsaturated images were interleaved for each TR period providing two data sets precisely co‐registrated. The total acquisition time was 20 min. MTR was calculated for each pixel according to the formula [(Mo−Ms)/Mo]×100 percent units (pu), where Mo and Ms represent signal intensities pre and post saturation pulse, respectively.
The following sets of images were also obtained in patients: dual fast spin echo (FSE) sequence (28 contiguous 5 mm axial slices, TR 2000 ms, TE 95 ms, NEX 1, matrix 256×256, FOV 240×180); and T1 weighted SE sequence (28 contiguous 5 mm axial slices, TR 540 ms, TE 20 ms, NEX 1, flip angle 90°, matrix 256×256, FOV 240×240) acquired before and 5 min after intravenous administration of triple dose gadolinium (Gd; 0.6 ml/kg of body weight; Magnevist, Schering, Berlin, Germany). MTI and FSE sequences were acquired on the same day. T1 weighted and T1 weighted Gd enhanced scans were acquired in a separate session from MTI (mean separation between sessions 20 days, median 13 days).
Image post processing
An automated segmentation procedure, using SPM99 software (Welcome Department of Cognitive Neurology, University College London, UK), was used to extract, starting from T2 weighted images, WM, GM, cerebrospinal fluid, and non‐brain tissues from the WB. A WB mask, which excludes CSF and other non‐brain parenchyma, was generated in SPM99 and applied to the calculated MTR map to obtain the WB MTR map. Then, a maximum likelihood algorithm, using the WM and GM probability outputs from SPM99, was used to segment the WB MTR map into WM and GM MTR maps. Macroscopic lesions were identified, contoured, and segmented using a reproducible semiautomated local thresholding technique (DispImage; Plummer, Department of Medical Physics and Bioengineering, University College London, UK) on the unsaturated MT proton density (PD) weighted images, T2 weighted FSE images, and T1 weighted Gd enhanced images. Lesions in PD, T2, T1, and Gd enhanced scans were used to calculate lesions loads. In addition, lesions in PD weighted scans were used to create a lesion mask. Where WM lesions were encountered in controls (five subjects, either non‐specific or thought likely to be due to incidental small vessel disease), the same methodology was applied in order to obtain the NABT of these subjects. The lesion mask was applied to the previously generated WB, WM, and GM MTR maps to obtain MTR maps of NABT, NAWM, and NAGM. Partial volume voxels were minimised with a 10 pu threshold and inner and outer voxel erosions of WM and GM.
SPM99 uses a histogram based segmentation procedure depending on voxel intensity that may be biased by the presence of abnormal tissue intensity.28 In patients with low lesion load, the segmentation is correct, but in patients with high lesions loads, it tends to include WM tissue in GM. This misclassification has the potential to alter the NAGM MTR values observed. Therefore, we decided to exclude from NAGM analysis those patients with T2 lesion loads above 50 ml. This may have reduced the chances of observing significant differences as the excluded patients were likely to display greater abnormality.
An MTR histogram generation algorithm was implemented for each NABT, NAWM, and NAGM. Inter subject variability in specific tissue volumes was adjusted for by normalising the MTR histograms. This was achieved by dividing the number of counts in each sampling bin by the total number of voxels for each tissue type. Normalisation was used to allow comparison of histograms from subjects with different brain volumes. From each normalised histogram, mean MTR (M), peak height (PH), peak location (PL), and the MTR value of the 25th, 50th, and 75th percentiles (P25, P50, P75) were measured. Coefficients of variation (COVs) for 18 healthy control subjects in scans performed 1 year apart were 0.56% for WB, 0.43% for WM, and 1.048% for GM. COVs for 14 patients in scans performed 1 month apart were 0.61% for WB, 0.60% for NABT, 0.69% for WM, 0.62% for NAWM, 0.84% for GM, and 0.85% for NAGM.
Statistical analysis
Statistical analysis was performed using SPSS 10.0 (Chicago, IL, USA). MTR parameters of NABT, NAWM, and NAGM were compared between patients and controls using general linear modelling including age and gender in the model to account for slight differences in these variables between the two groups. The Bonferroni correction for multiple comparisons was applied with a p value of 0.003 or less regarded as significant. Pearson's correlation coefficients were used to assess the presence of associations between MTR parameters and clinical and radiological variables. Spearman's rank correlation coefficient was used for EDSS comparisons. Two tailed t tests were used for comparison of radiological variables between clinical subgroups of PPMS (cord and non‐cord onset).
Results
Descriptive data
We studied 43 patients with PPMS (24 males and 19 females) with a mean age of 45.12±10.84 years (range 22–65 years) and a mean disease duration of 3.33±0.87 years (range 2–5 years). The median EDSS was 4.5 (range 3.0–7.0). Forty three controls (23 males and 20 females) were also scanned (mean age of 37.21±8.99 years; range 23–59 years).
Comparison of MTR parameters between patients and controls
There were significant differences between patients and controls in M, PL, and PH of NABT, NAWM, and NAGM (p⩽0.001) (table 1). Within the group of patients with PPMS, only NABT M was statistically different between cord (mean 34.228±1.120) and non‐cord (mean 33.138±1.619) presentation at onset (p = 0.028).
Table 1 Magnetisation transfer ratio (MTR) values for controls and patients.
| NABT | NAWM | NAGM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls (n = 43) | Patients (n = 43) | p value | Controls (n = 43) | Patients (n = 43) | p value | Controls (n = 43) | Patients (n = 39) | p value | |
| M | 35.174±0.358 | 34.026±1.279 | <0.001 | 38.238±0.399 | 36.938±2.290 | <0.001 | 32.254±0.470 | 31.358±0.776 | <0.001 |
| PH | 0.0113±0.001 | 0.0105±0.001 | <0.001 | 0.0201±0.002 | 0.0180±0.003 | <0.001 | 0.0125±0.001 | 0.0107±0.001 | <0.001 |
| PL | 37.598±0.376 | 36.688±1.307 | <0.001 | 38.470±0.448 | 37.570±1.089 | <0.001 | 33.181±0.348 | 32.731±0.616 | 0.001 |
| P25 | 21.163±1.393 | 21.379±2.216 | 0.872 | 23.384±1.837 | 23.095±2.476 | 0.328 | 20.572±1.195 | 20.733±1.228 | 0.851 |
| P50 | 32.184±2.795 | 32.628±4.410 | 0.857 | 32.786±2.485 | 32.398±2.914 | 0.160 | 30.967±2.355 | 31.321±2.448 | 0.881 |
| P75 | 43.186±4.216 | 43.586±6.783 | 0.990 | 42.156±3.547 | 41.674±3.969 | 0.144 | 41.340±3.544 | 41.882±3.679 | 0.894 |
Comparisons have been performed using general linear modelling adjusting for age and gender.
All values are presented as mean±standard deviation. M, mean MTR; NABT, normal appearing brain tissue; NAGM, normal appearing grey matter; NAWM, normal appearing white matter; P25, MTR at the 25th percentile; P50, MTR at the 50th percentile; P75, MTR at the 75th percentile; PH, peak height; PL, peak location.
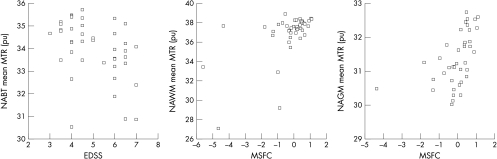
Clinical correlations of MTR parameters
Weak to moderate correlations were found between MTR parameters in NABT and the two clinical measures: EDSS (table 2 and fig 1) and MSFC (table 2). Of the constituents of the MSFC, the z‐9HPT correlated more strongly with NABT than either NAGM or NAWM, and the z‐TWT correlated more strongly with NAWM MTR parameters than with NAGM, while the z‐PASAT correlated more strongly with NAGM MTR parameters than with NAWM.
Table 2 Correlations between magnetisation transfer ratio (MTR) parameters and disability measurements.
| EDSS | MSFC | z‐9HPT | z‐PASAT | z‐TWT | ||
|---|---|---|---|---|---|---|
| NABT (n = 43) | M | −0.46** | 0.53** | 0.65** | 0.38* | −0.35* |
| PH | −0.51** | 0.43** | 0.81** | 0.39** | −0.18 | |
| PL | −0.35* | 0.44** | 0.50** | 0.32* | −0.30 | |
| P25 | −0.30* | 0.22 | −0.01 | 0.13 | −0.23 | |
| P50 | −0.29 | 0.21 | −0.02 | 0.12 | −0.22 | |
| P75 | −0.23 | 0.19 | −0.06 | 0.16 | −0.20 | |
| NAWM (n = 43) | M | −0.34* | 0.58** | 0.42** | 0.31* | −0.49** |
| PH | −0.33* | 0.49** | 0.61** | 0.33* | −0.32* | |
| PL | −0.31* | 0.51** | 0.51** | 0.33* | −0.37* | |
| P25 | −0.48** | 0.48** | 0.34* | 0.23 | −0.41** | |
| P50 | −0.43** | 0.42** | 0.33* | 0.28 | −0.34* | |
| P75 | −0.30* | 0.32* | 0.27 | 0.26 | −0.24 | |
| NAGM (n = 39) | M | −0.34* | 0.51** | 0.54** | 0.48** | −0.22 |
| PH | −0.40* | 0.49** | 0.65** | 0.39* | −0.19 | |
| PL | −0.18 | 0.57** | 0.34* | 0.31 | −0.43** | |
| P25 | −0.34* | 0.33* | 0.22 | 0.23 | −0.22 | |
| P50 | −0.33* | 0.32 | 0.20 | 0.23 | −0.21 | |
| P75 | −0.31 | 0.31 | 0.19 | 0.23 | −0.21 |
Pearson's correlation coefficients were used except for the EDSS (Spearman's rank correlation coefficient). EDSS, Expanded Disability Status Scale; M, mean MTR; MSFC, Multiple Sclerosis Functional Composite; NABT, normal appearing brain tissue; NAGM, normal appearing grey matter; NAWM, normal appearing white matter; P25, MTR at the 25th percentile; P50, MTR at the 50th percentile; P75, MTR at the 75th percentile; PH, peak height; PL, peak location; z‐9HPT, z score for the Nine‐Hole Peg Test; z‐PASAT, z score for the Paced Auditory Serial Addition Test; z‐TWT, z score for the Timed Walk Test.
Significance: *p<0.05; **p<0.01.
Figure 1 Normal appearing brain tissue (NABT) mean magnetisation transfer ratio (MTR) plotted against EDSS. Normal appearing white matter (NAWM) and normal appearing grey matter (NAGM) mean MTR plotted against Multiple Sclerosis Functional Composite (MSFC).
MRI correlations of MTR parameters
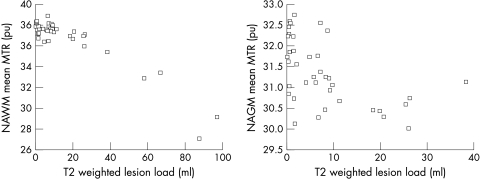
Strong correlations were found between MTR parameters of NABT and NAWM, and PD, T2, and T1 lesion loads and less so for NAGM parameters (table 3 and fig 2). Enhancing lesion number correlated with MTR parameters in NABT and NAWM but not in NAGM.
Table 3 Correlations between magnetisation transfer ratio (MTR) parameters and lesion loads.
| PD | T2 | T1 | GLN | GLV | ||
|---|---|---|---|---|---|---|
| NABT (n = 43) | M | −0.86** | −0.79** | −0.69** | −0.47** | −0.20 |
| PH | −0.63** | −0.59** | −0.51** | −0.33* | −0.25 | |
| PL | −0.84** | −0.83** | −0.73** | −0.44** | −0.18 | |
| P25 | −0.03 | 0.00 | −0.08 | −0.08 | −0.18 | |
| P50 | −0.01 | 0.01 | −0.07 | −0.07 | −0.07 | |
| P75 | 0.02 | 0.04 | −0.05 | −0.05 | −0.14 | |
| NAWM (n = 43) | M | −0.88** | −0.94** | −0.91** | −0.58** | −0.25 |
| PH | −0.73** | −0.81** | −0.82** | −0.42** | −0.33* | |
| PL | −0.85** | −0.86** | −0.81** | −0.43** | −0.17 | |
| P25 | −0.41** | −0.45** | −0.46** | −0.34* | −0.08 | |
| P50 | −0.46** | −0.43** | −0.38* | −0.34* | −0.12 | |
| P75 | −0.41** | −0.35* | −0.27 | −0.28 | −0.12 | |
| NAGM (n = 39) | M | −0.60** | −0.53** | −0.57** | −0.10 | 0.08 |
| PH | −0.47** | −0.32 | −0.55** | −0.12 | −0.08 | |
| PL | −0.37* | −0.33* | −0.34* | −0.14 | −0.01 | |
| P25 | −0.23 | −0.29 | −0.39* | −0.24 | −0.29 | |
| P50 | −0.21 | −0.26 | −0.37* | −0.23 | −0.28 | |
| P75 | −0.20 | −0.26 | −0.37* | −0.22 | −0.28 |
Pearson's correlation coefficients were used in all comparisons. GLN, gadolinium enhanced lesion number; GLV, gadolinium enhanced lesion volume; M, mean MTR; NABT, normal appearing brain tissue; NAGM, normal appearing grey matter; NAWM, normal appearing white matter; P25, MTR at the 25th percentile; P50, MTR at the 50th percentile; P75, MTR at the 75th percentile; PD, proton density weighted lesion load; PH, peak height; PL, peak location; T1, T1 weighted lesion load; T2, T2 weighted lesion load.
Significance: *p<0.05; **p<0.01.
Figure 2 Normal appearing white matter (NAWM) and normal appearing grey matter (NAGM) mean magnetisation transfer ratio (MTR) plotted against T2 weighted lesion load.
Discussion
This study confirms the existence of MTR abnormalities in PPMS. We have now shown that these abnormalities can be detected in the early stages of the disease, affect both NAWM and NAGM, and are related to clinical status and abnormalities on conventional MRI.
MTR abnormalities of NABT are present in early disease
Previous studies in PPMS,21,22,23 using different methodologies and assessing different tissue fractions, have also found differences between patients and controls in some MTR parameters. Studies using histogram analysis found differences in some WB,22 NAWM,21 and NAGM21 MTR parameters, while studies analysing regions of interest found MTR differences in the corpus callosum, the frontal lobes, and the centrum semiovale.23 In our study, we have found consistent differences in the most sensitive and informative MTR parameters (M, PL, and PH) in all the normal appearing brain tissues (NABT, NAWM, and NAGM), suggesting quite extensive change and underlining the sensitivity of MTR measurements in detecting subtle change. These MTR abnormalities observed between patients and controls are significantly larger than the values estimates by the reported COVs, indicating that these differences are partly due to group effect (controls or patients) and not only to the technique used or to a time effect.
MTR abnormalities of NABT are associated with clinical status
This study suggests that abnormalities in both NAWM and NAGM are relevant to the development of disability in early PPMS. Patients with PPMS take on average 8 years to progress in disability to DSS (Disability Status Score) 6, and then a further 18 years to progress from DSS 6 to 8.29 It is possible that in the early years of the disease, when the greatest changes in disability are occurring, the pathological changes are correspondingly greater. The mean disease duration of patients in the present study was 3.3 years in comparison to mean durations of 6, 7, and 8 years in previous studies.21,22,23
In the present study, weak to moderate correlations were found with disability as measured by the EDSS and the MSFC. These were mainly between M, PH, and PL MTR and were observed in NABT, NAWM, and NAGM. This is in contrast to previous studies, where correlations between WB MTR histogram parameters and the EDSS were either weak21,23 or non‐existent.22 The differences in results between the present and previous studies may relate to the use of different methodologies (using regions of interest only some parts of the brain are analysed, while using histogram analysis the entire brain is studied) or, more interestingly, might reflect a greater clinical relevance of MRI measurable pathological processes in early disease, as mentioned earlier. EDSS correlates more strongly with NABT than with NAWM and NAGM. This may indicate widespread and clinically relevant tissue damage in early PPMS. Our data also showed that MTR parameters correlate more strongly with MSFC and its components than with EDSS. We found interesting correlations between the different components of the MSFC and the different brain tissues suggesting tissue specific effects on disability (table 2): while 9HPT performance relates similarly to both NAGM and NAWM, poorer PASAT performance is more related to GM changes and slower walking times (TWT) relate strongly to changes in WM.
MTR abnormalities of NABT are strongly associated with lesion load
We found strong correlations between lesion load and a number of MTR histogram parameters in both NAWM and NAGM, but mainly in the former. A previous study found weak correlations between T2 and T1 lesion loads and mean NABT.30 The weakness of this association was interpreted as suggesting that NABT pathology in PPMS might not merely reflect wallerian degeneration of axons traversing macroscopic lesions, but might, in addition, relate to the occurrence of subtle pathology, independent of macroscopic lesions. Our results seem to suggest that a greater association between NABT MTR abnormalities and the process of lesion formation is present in early disease. A weak to moderate correlation between NAWM and gadolinium enhancing lesion number was also found. This relationship supports an association between inflammation and MTR abnormalities in NAWM. MTR correlations with lesion load were strongest in NAWM, where the lesions are found on conventional MRI, but moderate correlations were also found in NAGM. This might be explained by lesion formation in WM causing retrograde damage in the GM or by a pathological process affecting both NAWM and NAGM indiscriminately.
In summary, this study supports the existence of a diffuse disease process operating in the early stages of PPMS which affects both NAWM and NAGM, and which relates to both disability and lesion presence. Further longitudinal follow up study of this cohort will seek to extend these findings, assess the value of MTI as a marker of prognosis, and examine the relationship of MTR to other pathologically specific MRI techniques.
Acknowledgements
The authors would like to thank the subjects who kindly agreed to take part in this study.
Abbreviations
COVs - coefficients of variation
DSS - Disability Status Score
EDSS - Expanded Disability Status Scale
FOV - field of view
FSE - fast spin echo
Gd - gadolinium
GM - grey matter
M - mean MTR
MRI - magnetic resonance imaging
MS - multiple sclerosis
MSFC - Multiple Sclerosis Functional Composite
MTI - magnetisation transfer imaging
MTR - magnetisation transfer ratio
NABT - normal appearing brain tissue
NAGM - normal appearing grey matter
NAWM - normal appearing white matter
NEX - number of excitations
P25 - MTR value of the 25th percentile
P50 - MTR value of the 50th percentile
P75 - MTR value of the 75th percentile
PD - proton density
PH - peak height
PL - peak location
PPMS - primary progressive multiple sclerosis
pu - percent unit
RRMS - relapsing‐remitting multiple sclerosis
SE - spin echo
TE - echo time
TR - repetition time
WB - whole brain
WM - white matter
Footnotes
The authors would like to acknowledge the support of the Wellcome Trust (GTI), the Spanish Ministry of Health (JSG, BEFI #02/9115), and the MS Society of Great Britain and Northern Ireland
Competing interests: none declared
Ethics approval: this study was approved by the Joint Medical Ethics Committee of the National Hospital for Neurology and Neurosurgery and Institute of Neurology, London, UK. Written informed consent was obtained from all subjects before they were included in the study
References
- 1.Thompson A J, Polman C H, Miller D H.et al Primary progressive multiple sclerosis. Brain 1997120(6)1085–1096. [DOI] [PubMed] [Google Scholar]
- 2.Allen I V, McKeown S R. A histological, histochemical and biochemical study of the macroscopically normal white matter in multiple sclerosis. J Neurol Sci 197941(1)81–91. [DOI] [PubMed] [Google Scholar]
- 3.Trapp B D, Peterson J, Ransohoff R M.et al Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998338(5)278–285. [DOI] [PubMed] [Google Scholar]
- 4.Kidd D, Barkhof F, McConnell R.et al Cortical lesions in multiple sclerosis. Brain 1999122(1)17–26. [DOI] [PubMed] [Google Scholar]
- 5.Chard D T, Griffin C M, McLean M A.et al Brain metabolite changes in cortical grey and normal‐appearing white matter in clinically early relapsing‐remitting multiple sclerosis. Brain 2002125(Pt 10)2342–2352. [DOI] [PubMed] [Google Scholar]
- 6.Filippi M. MRI‐clinical correlations in the primary progressive course of MS: new insights into the disease pathophysiology from the application of magnetization transfer, diffusion tensor, and functional MRI. J Neurol Sci 2003206(2)157–164. [DOI] [PubMed] [Google Scholar]
- 7.Grossman R I. Magnetization transfer in multiple sclerosis. Ann Neurol 199436(suppl)S97–S99. [DOI] [PubMed] [Google Scholar]
- 8.Lexa F J, Grossman R I, Rosenquist A C. Detection of early axonal degeneration in the mammalian central nervous system by magnetization transfer techniques in magnetic resonance imaging. Ann N Y Acad Sci 1993679336–340. [DOI] [PubMed] [Google Scholar]
- 9.Lexa F J, Grossman R I, Rosenquist A C. Dyke Award paper. MR of wallerian degeneration in the feline visual system: characterization by magnetization transfer rate with histopathologic correlation, AJNR Am J Neuroradiol 199415(2)201–212. [PMC free article] [PubMed] [Google Scholar]
- 10.Dousset V, Armand J P, Lacoste D.et al Magnetization transfer study of HIV encephalitis and progressive multifocal leukoencephalopathy. Groupe d'Epidemiologie Clinique du SIDA en Aquitaine. AJNR Am J Neuroradiol 199718(5)895–901. [PMC free article] [PubMed] [Google Scholar]
- 11.Silver N C, Barker G J, MacManus D G.et al Decreased magnetisation transfer ratio due to demyelination: a case of central pontine myelinolysis. J Neurol Neurosurg Psychiatry 199661(2)208–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dousset V, Grossman R I, Ramer K N.et al Experimental allergic encephalomyelitis and multiple sclerosis: lesion characterization with magnetization transfer imaging. Radiology 1992182(2)483–491. [DOI] [PubMed] [Google Scholar]
- 13.Dousset V, Brochet B, Vital A.et al Lysolecithin‐induced demyelination in primates: preliminary in vivo study with MR and magnetization transfer. AJNR Am J Neuroradiol 199516(2)225–231. [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta R C, Pike G B, Enzmann D R. Measure of magnetization transfer in multiple sclerosis demyelinating plaques, white matter ischemic lesions, and edema. AJNR Am J Neuroradiol 199617(6)1051–1055. [PMC free article] [PubMed] [Google Scholar]
- 15.van Waesberghe J H, Kamphorst W, De Groot C J.et al Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol 199946(5)747–754. [DOI] [PubMed] [Google Scholar]
- 16.Silver N C, Lai M, Symms M R.et al Serial magnetization transfer imaging to characterize the early evolution of new MS lesions. Neurology 199851(3)758–764. [DOI] [PubMed] [Google Scholar]
- 17.Filippi M, Iannucci G, Tortorella C.et al Comparison of MS clinical phenotypes using conventional and magnetization transfer MRI. Neurology 199952(3)588–594. [DOI] [PubMed] [Google Scholar]
- 18.Filippi M, Campi A, Dousset V.et al A magnetization transfer imaging study of normal‐appearing white matter in multiple sclerosis. Neurology 199545(3 Pt 1)478–482. [DOI] [PubMed] [Google Scholar]
- 19.Griffin C M, Chard D T, Parker G J.et al The relationship between lesion and normal appearing brain tissue abnormalities in early relapsing remitting multiple sclerosis. J Neurol 2002249(2)193–199. [DOI] [PubMed] [Google Scholar]
- 20.Filippi M, Rocca M A, Martino G.et al Magnetization transfer changes in the normal appearing white matter precede the appearance of enhancing lesions in patients with multiple sclerosis. Ann Neurol 199843(6)809–814. [DOI] [PubMed] [Google Scholar]
- 21.Dehmeshki J, Chard D T, Leary S M.et al The normal appearing grey matter in primary progressive multiple sclerosis: a magnetisation transfer imaging study. J Neurol 2003250(1)67–74. [DOI] [PubMed] [Google Scholar]
- 22.Dehmeshki J, Silver N C, Leary S M.et al Magnetisation transfer ratio histogram analysis of primary progressive and other multiple sclerosis subgroups. J Neurol Sci 2001185(1)11–17. [DOI] [PubMed] [Google Scholar]
- 23.Leary S M, Silver N C, Stevenson V L.et al Magnetisation transfer of normal appearing white matter in primary progressive multiple sclerosis. Mult Scler 19995(5)313–316. [DOI] [PubMed] [Google Scholar]
- 24.Thompson A J, Montalban X, Barkhof F.et al Diagnostic criteria for primary progressive multiple sclerosis: a position paper. Ann Neurol 200047(6)831–835. [PubMed] [Google Scholar]
- 25.Kurtzke J F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 198333(11)1444–1452. [DOI] [PubMed] [Google Scholar]
- 26.Cutter G R, Baier M L, Rudick R A.et al Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 1999122(5)871–882. [DOI] [PubMed] [Google Scholar]
- 27.Barker G J, Tofts P S, Gass A. An interleaved sequence for accurate and reproducible clinical measurement of magnetization transfer ratio. Magn Reson Imaging 199614(4)403–411. [DOI] [PubMed] [Google Scholar]
- 28.Chard D T, Parker G J, Griffin C M.et al The reproducibility and sensitivity of brain tissue volume measurements derived from an SPM‐based segmentation methodology. J Magn Reson Imaging 200215(3)259–267. [DOI] [PubMed] [Google Scholar]
- 29.Cottrell D A, Kremenchutzky M, Rice G P.et al The natural history of multiple sclerosis: a geographically based study. 5. The clinical features and natural history of primary progressive multiple sclerosis. Brain 1999122(Pt 4)625–639. [DOI] [PubMed] [Google Scholar]
- 30.Rovaris M, Bozzali M, Santuccio G.et al In vivo assessment of the brain and cervical cord pathology of patients with primary progressive multiple sclerosis. Brain 2001124(Pt 12)2540–2549. [DOI] [PubMed] [Google Scholar]