Abstract
Background
A previous cross sectional study found over‐representation of a postural instability gait difficulty (PIGD) motor subtype in Parkinson's disease patients with dementia (PDD) and dementia with Lewy bodies (DLB), compared with Parkinson's disease (PD).
Aims
(1) To examine rates of cognitive and motor decline over two years in PD (n = 40), PDD (n = 42) and DLB (n = 41) subjects, compared with age matched controls (n = 41), (2) to record whether motor phenotypes of PD, PDD, and DLB subjects changed during the study, (3) to find out if cognitive and motor decline in PD was associated with baseline motor subtype, and (4) to report the incidence of dementia in PD patients in relation to baseline motor subtype.
Results
Most of PDD and DLB participants were PIGD subtype at baseline assessment. In the non‐demented PD group, tremor dominant (TD) and PIGD subtypes were more evenly represented. Cognitive decline over two years was greater in PDD and DLB groups (mean decline in MMSE −4.5 and −3.9, respectively), compared with PD (−0.2) and controls (−0.3). There was an association between PIGD subtype and increased rate of cognitive decline within the PD group. Of 40 PD patients, 25% of the 16 PIGD subtype developed dementia over two years, compared with none of the 18 TD or six indeterminate phenotype cases (χ2 = 6.7, Fisher's exact test p<0.05).
Conclusion
A PIGD motor subtype is associated with a faster rate of cognitive decline in PD and may be considered a risk factor for incident dementia in PD.
Keywords: Lewy body disease, dementia, parkinsonism, motor subtype, progression
The cumulative incidence of dementia in Parkinson's disease (PD) may be as high as 80%.1 Age is the main predictor of increased risk for dementia in PD (PDD). More rapid cognitive decline in PD has also, however, been associated with severity of motor symptoms2 and motor subtype.3 In particular, motor symptoms believed to be mediated by non‐dopaminergic mechanisms, such as gait, speech, and postural control, were recently associated with accelerated decline in cognition.4 Neurodegeneration within the cholinergic system is likely to mediate non‐dopaminergic motor features in PDD and dementia with Lewy bodies (DLB), as well as having a significant role in determining the cognitive and neuropsychiatric symptoms in these disorders.5
We previously found in a cross sectional study a significant over‐representation of a so called postural instability gait difficulty (PIGD) subtype in patients with PDD (88%) and DLB (69%), compared with PD (38%).6 In the second group, tremor dominant (TD) and PIGD subtypes were more evenly represented.
In this study, we followed up a cohort comprising PD, PDD, DLB, and age matched controls over two years. The aims were: (1) to examine the rates of cognitive and motor decline over two years in the different patient groups, compared with controls, (2) to monitor whether motor subtypes of PD, PDD, and DLB subjects changed during the course of the study, (3) to find out if the amount of cognitive and motor decline in PD was associated with baseline PIGD motor subtype, and (4) to report the incidence of dementia in PD patients in relation to baseline motor subtype. We hypothesised that there would be an association with cognitive decline or dementia, or both, in those PD patients with a baseline PIGD subtype.
Methods
Consecutive patients fulfilling entry criteria were recruited over 24 months from local dementia and movement disorder clinics in Newcastle and Sunderland. Age matched controls were mainly spouses of patients involved in the study. Eighty one subjects with a provisional diagnosis of PD were screened for the study. Forty of these PD patients agreed to participate, met Queen Square Brain Bank criteria,7 were aged 65 or over and taking L‐dopa monotherapy (to avoid potential confounding neuropsychiatric effects of other dopaminergic agents), and scored more than 24 on the mini‐mental state examination (MMSE).8 Of the 41 PD subjects who were not recruited, 28 refused participation, one was too frail, and 12 others did not meet study criteria. Forty two PDD patients were recruited and met the same criteria as the PD group but, in contrast, fulfilled diagnostic and statistical manual (DSM‐IV) criteria for dementia and had a MMSE score less than 24. They also had to have historical evidence of visual hallucinations and/or fluctuating cognition with variability in attention and alertness. A further 33 subjects with a provisional diagnosis of PDD were screened for participation, of whom 14 declined to participate, one died, three were too frail, and 15 others did not meet study entry criteria. Eighty one subjects with a provisional diagnosis of DLB were screened. Forty one DLB patients were recruited (34 with probable DLB and seven with possible DLB) and were diagnosed according to consensus criteria.9 Of those DLB subjects not recruited, 21 refused to participate and 19 did not meet study entry criteria. On entry into the study, two PDD and two DLB patients were taking atypical antipsychotic drugs in low dose, while six PDD and 13 DLB patients were taking a cholinesterase inhibitor.
All subjects were assessed at entry to the study (baseline) and annually thereafter. The two year follow up visit was performed a mean (SD) of 24 (2) months after baseline visit. At each visit, activities of daily living (ADL) were recorded using the unified Parkinson's disease rating scale (UPDRS part II) and extrapyramidal motor features rated using the UPDRS part III.10 Items derived from these two subscales permit determination of PD subtype, according to the method proposed by Jankovic and colleagues.11 The subtypes are referred to as TD, PIGD, or indeterminate (IND). All patients were assessed throughout while taking their normal drug treatment and L‐dopa was not withheld for motor examination. As well as the MMSE, cognitive function was assessed using the Cambridge cognitive examination (CAMCOG).12 Depression was assessed using the geriatric depression scale (GDS).13 The study received approval from the local research ethics committee.
Statistical analysis
Outcome measures were examined both graphically and using the Kolmogorov‐Smirnov test to establish whether they were approximately normally distributed. One way analysis of variance with Games‐Howell post hoc tests was used to compare age across groups. The χ2 test was used to compare the sex and motor phenotype distributions. Mann‐Whitney tests and Kruskall‐Wallis tests were applied for other comparisons of baseline characteristics where data were not normally distributed.
One way analysis of variance with Games‐Howell post hoc tests was used to compare changes in CAMCOG, MMSE, and UPDRS III total scores across the patient groups over two years. Forward stepwise linear regression analysis was undertaken to examine whether there was an association between each of the dependent (outcome) variables (change in MMSE, CAMCOG, and UPDRS III scores over two years) and the presence of the PIGD subtype at baseline (independent variable) in PD subjects. For this part of the analysis PIGD was coded as 1 and TD or IND subtypes were coded as 0. Other covariates included were baseline GDS depression score and baseline CAMCOG total, as in our previous paper these differed significantly between TD and PIGD PD subjects at initial assessment. SPSS (version 13) was used for all statistical analyses.
Results
Demographics and baseline motor subtype
Table 1 shows the demographics of the three patient groups and the control group. The groups were well matched for age (one way analysis of variance: F = 2.0, p = 0.124), although there was a non‐significant trend for more male patients in the PD and PDD groups compared with the control and DLB groups (χ2 = 4.4, Fishers exact test, p = 0.054). Mean levels of cognitive impairment differed across the four groups at baseline (for MMSE: χ2 = 110, p<0.001; for CAMCOG: χ2 = 115, p<0.001). Controls were least impaired followed by PD, PDD, and DLB groups. PDD patients had a longer duration of extrapyramidal symptoms compared with the PD and DLB groups (mean 8.5 years v 4.3 and 2.0 years, respectively, p <0.001). At baseline, 29 PD, 40 PDD, and seven DLB subjects were taking L‐dopa; there was no significant difference between the mean dose of L‐dopa in each group (one way analysis of variance: F = 0.731, p = 0.49). The PIGD motor subtype was significantly over‐represented in the PDD (83% of all patients) and DLB (73%) groups compared with the PD group (40%) (χ2 = 23.8, p<0.001, for PDD and DLB combined v PD).
Table 1 Baseline demographic, cognitive, and motor characteristics.
| Characteristic | Control (n = 41) | PD (n = 40) | PDD (n = 42) | DLB (n = 41) |
|---|---|---|---|---|
| Mean age, y (SD) | 75.2 (6.8) | 75.4 (5.7) | 73.1 (5.8) | 76.4 (6.8) |
| Men, n (%) | 22 (54) | 30 (75) | 27 (64) | 22 (54) |
| Duration of cognitive symptoms at baseline, y (SD) | NA | NA | 2.8 (2.4) (n = 38) | 2.5 (1.9) (n = 39) |
| Mean MMSE (SD) (maximum score = 30) | 28 (2) | 27 (2) | 19 (6) | 16 (5) |
| Mean total CAMCOG (SD) (maximum score = 105) | 94 (4) | 89 (7) | 64 (15) | 59 (14) (n = 38) |
| Duration of parkinsonism at baseline, y (SD) | NA | 4.3 (4.2) | 8.5 (6.4) | 2.0 (1.5) (n = 16) |
| Baseline mean L‐dopa dose mg (SD), number of cases | NA | 435 (309) (n = 29) | 433 (227) (n = 40) | 307 (249) (n = 7) |
| Two year mean L‐dopa dose mg (SD), number of cases | NA | 494 (290) (n = 34) | 460 (242) (n = 23) | 310 (185) (n = 18) |
| Motor subtype: | NA | |||
| PIGD, n (%) | 16 (40) | 35 (83) | 30 (73) | |
| TD, n (%) | 18 (45) | 3 (7) | 4 (10) | |
| Indeterminate, n (%) | 6 (15) | 4 (10) | 7 (17) |
NA, not applicable.
Cognitive and motor decline
At two years' follow up, three controls, five PD (including one death), 18 PDD (including nine deaths), and 10 DLB (including four deaths) patients were lost to follow up. Demented subjects were significantly more likely to be lost to follow up by two years than non‐demented subjects (Fisher's exact test: p<0.001, OR = 4.7, CI = 2.0 to 11.0). Not all remaining DLB and PDD participants were able to complete the MMSE, CAMCOG, and UPDRS III tests at two years. The main reasons for missing data were sleepiness, inability to engage, being mute, or being bed/wheelchair bound.
Table 2 shows the mean decline in cognition across all groups, and according to baseline motor subtype. Mean decline in MMSE score over two years for each group was: controls = 0.3, PD = 0.2, PDD = 4.5, and DLB = 3.9 points (one way analysis of variance; F = 8.2, p<0.001). Games‐Howell post hoc tests showed that there was a significant difference between the two dementia groups in comparison with both controls and PD patients (p<0.05) while there was no difference between PDD and DLB groups (p = 0.987) or between the control and PD groups (p = 0.997). Mean total CAMCOG score did not decline over two years in controls but fell in the PD, PDD, and DLB groups by 2, 11, and 10 points, respectively. There was a significant difference in the cognitive decline across the groups (F = 9.5 p<0.001), with post hoc tests showing a significant difference between each of the two dementia groups and controls (p<0.05) but not between the dementia and PD groups (PD v DLB, p = 0.059; PD v PDD, p = 0.102). Additionally, there was no difference between the controls and PD subjects (p = 0.367) or between PDD and DLB groups (p = 0.987). Over the two year study period, cholinesterase inhibitor drug use increased in the DLB group from 13 patients at baseline to 22 patients, and in the PDD group from six patients at baseline to 20 patients at two years.
Table 2 Cognitive and motor change between baseline and two years in patient groups, according to baseline motor subtype.
| Group (n) | Controls (n = 38) | PD (n = 35) | PDD (n = 24) | DLB (n = 31) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MMSE | −0.3 (2.0) | −0.2 (2.9) | −4.5 (7.0) | −3.9 (5.2) | ||||||
| CAMCOG | 1.1 (6.9) | −1.8 (7.8) | −11.0 (14.2) | −9.5 (9.9) | ||||||
| UPDRS III | −1.6 (2.5) | −5.1 (13.4) | −9.7 (13.8) | −11.3 (14.6) | ||||||
| Phenotype (n) | IND (5) | PIGD (14) | TD (16) | IND (2) | PIGD (21) | TD (1) | IND (6) | PIGD (22) | TD (3) | |
| MMSE (n) | 0.2 (1.9) n = 5 | −1.8 (3.3) n = 14 | 1.1 (2.0) n = 16 | −1.0 (0.0) n = 2 | −5.1 (7.3) n = 20 | 0.0 n = 1 | −5.3 (4.6) n = 6 | −4.3 (4.9) n = 17 | 1.3 (6.5) n = 3 | |
| CAMCOG (n) | 1.0 (2.8) n = 5 | −6.1 (9.7) n = 14 | 1.2 (4.9) n = 16 | 1.5 (7.8) n = 2 | −11.7 (14.1) n = 13 | −27.0 n = 1 | −10.0 (9.8) n = 5 | −11.1 (7.8) n = 7 | −5.0 (16.5) n = 3 | |
| UPDRS III (n) | 1.0 (15.0) n = 5 | −8.9 (14.3) n = 14 | −3.6 (11.9) n = 16 | −10.0 (7.1) n = 2 | −9.8 (14.9) n = 18 | −7.0 n = 1 | −8.3 (12.1) n = 6 | −10.6 (16.7) n = 11 | −19.7 (11.2) n = 3 | |
Values are shown as means (SD). IND, indeterminate subtype. Negative numbers represent a worsening and positive numbers represent an improvement, compared with baseline values.
Mean UPDRS III scores deteriorated over the two year follow up in all patient groups, with decline in the PDD and DLB being more rapid (mean decline; controls = 1.6, PD = 5.1, PDD = 9.7 and DLB = 11.3 points, one way analysis of variance; F = 4.1, p = 0.008). Post hoc testing confirmed a significant difference between controls and DLB subjects (p = 0.04) and a borderline significant difference between the control and PDD groups (p = 0.07). The deterioration in motor scores occurred despite the use of L‐dopa in an additional eight PD, one PDD, and 14 DLB subjects, compared with baseline. Table 1 illustrates the mean L‐dopa dose taken at two years; there was no significant change in the dose taken across the three groups over this period (one way analysis; F = 2.9, p = 0.063).
There was no significant difference in rate of cognitive and motor decline between PD and PDD patients of short and long disease duration (groups determined by dichotomising about the mean disease duration; data not shown).
Change in motor phenotype over two years
In 66% of the 90 PD, PDD, and DLB subjects still available for assessment at two years, motor phenotype was the same at two years as it had been at baseline. Although no PIGD PD subjects converted to a TD subtype, four of six indeterminate PD patients were re‐classified as a PIGD subtype and four of 18 TD patients were re‐classified as an indeterminate subtype at two years.
Within the DLB group, one of four TD DLB subjects had converted to PIGD before withdrawal at one year and of seven indeterminate subjects, two had converted to TD and two to PIGD at two years.
Within the PDD group, only one of 35 PIGD subjects converted to TD at two years. Of three TD PDD patients at baseline, two evolved to a PIGD subtype while the third died during follow up. Of four indeterminate PDD subjects at baseline, two were classified as TD at two years.
Cognitive decline, incident dementia, and baseline motor subtype in PD patients
Univariate linear regression analyses showed significant associations between change in MMSE score over two years and the presence/absence of the PIGD motor phenotype as well as baseline total CAMCOG score in PD subjects (table 3). There was no significant association between change in MMSE score and baseline GDS score. The final multivariate model showed that the magnitude of decline in the MMSE score of PIGD PD patients was greater than that of non‐PIGD PD subjects and that this decline was exacerbated by a lower baseline total CAMCOG score (b = −2.29, 95%CI: −4.13 to −0.46, p = 0.016).
Table 3 Results of forward stepwise linear regression analysis for predictors of change in cognition and motor function in PD subjects.
| Outcome measure | Explanatory variable | Univariate models | Final multivariate models | ||||
|---|---|---|---|---|---|---|---|
| b | 95%CI | p | b | 95%CI | p | ||
| Change in MMSE score | Presence of a PIGD subtype | −2.64 | −4.45 to −0.84 | 0.005 | −2.29 | −4.13 to −0.46 | 0.016 |
| Baseline total CAMCOG score | 0.15 | 0.00 to 0.30 | 0.044 | ||||
| Baseline total GDS score | 0.18 | −0.18 to 0.54 | 0.316 | ||||
| Change in total CAMCOG score | Presence of a PIGD subtype | −7.29 | −12.20 to −2.37 | 0.005 | −7.29 | −12.20 to −2.37 | 0.005 |
| Baseline total CAMCOG score | 0.14 | −0.28 to 0.56 | 0.490 | ||||
| Baseline total GDS score | 0.36 | −0.63 to 1.35 | 0.462 | ||||
| Change in total UPDRS III score | Presence of a PIGD subtype | −6.45 | −15.73 to 2.82 | 0.166 | |||
| Baseline total CAMCOG score | 0.60 | −0.09 to 1.29 | 0.088 | ||||
| Baseline total GDS score | −0.88 | −2.57 to 0.80 | 0.294 | ||||
Univariate linear regression analyses showed a significant association between change in total CAMCOG score over two years and the presence/absence of the PIGD motor phenotype (b = −7.29, 95%CI: −12.20 to −2.37, p = 0.005). Thus, PD patients classified as PIGD at baseline had a greater decline in cognitive function. There was no significant association between change in total CAMCOG score and baseline GDS score or baseline total CAMCOG score.
Linear regression did not show any significant association between the change in UPDRS III score over two years and presence/absence of the PIGD phenotype in PD subjects (b = −6.45 95% CI = −15.7 to 2.8, p = 0.166). Also, there were no significant associations between change in UPDRS III scores and baseline GDS and total CAMCOG scores.
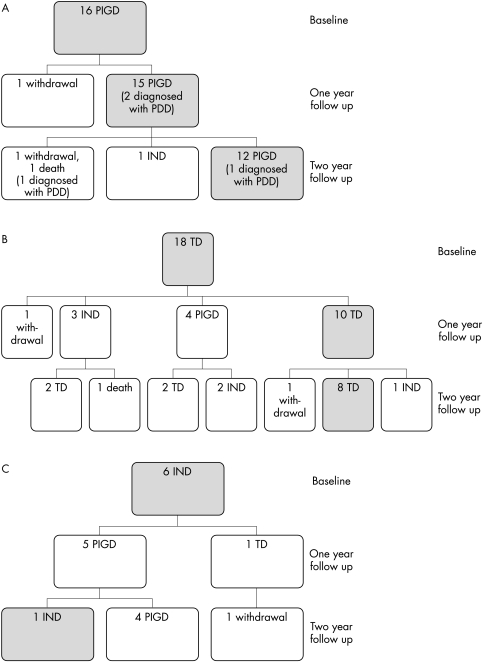
Figure 1 (A–C) summarises the motor phenotype changes over two years for the 40 PD patients who entered the study. Of the 16 PIGD patients at baseline, four (25%) developed dementia during the two year study period, compared with none in the combined TD and IND groups (χ2 = 6.7, Fisher's exact test p<0.05).
Figure 1 The motor phenotypes of non‐demented PD subjects and how they changed over two years (A) Phenotype changes in PD subjects who were PIGD at baseline (cases who converted to PDD are noted). (B) Phenotype changes in PD subjects who were TD at baseline. (C) Phenotype changes in PD subjects who were IND at baseline.
Discussion
The main outcome of this study is that in PD a PIGD motor subtype is generally associated with a more rapid rate of cognitive decline than a TD or IND subtype. Furthermore, incident dementia occurs more commonly in the PIGD subtype of PD, compared with indeterminate and TD subtypes.
The mean annual rates of cognitive decline, using the MMSE, in our study for PD and PDD are similar to those reported by Aarsland and colleagues.4 Those authors determined an annual decline in MMSE of 2.3 for PDD, in comparison with our figure of 2.25. Mean cognitive decline in non‐demented PD groups was negligible in both studies, at less than 0.1 points per annum. In contrast with the Aarsland study, we also studied DLB subjects, who displayed a similar annual MMSE decline to PDD of nearly two points. This is somewhat less than the annualised decline in MMSE noted by other studies for DLB, where mean values of 4.314 and 5.815 have been reported. These inter‐study differences cannot easily be explained by age of the DLB subjects or in their disease duration, although there were large standard deviations for the mean values presented in all studies. Furthermore, the MMSE may be a rather insensitive instrument for reflecting cognitive decline in DLB (and also in PDD), where executive deficits may be prominent. The mean decline in CAMCOG scores in our study was also similar for both PDD (11 points over two years) and DLB (10 points) groups, suggesting a degree of internal consistency in the data.
Annual rate of motor decline in PD, as evidenced by a worsening of nearly 2.5 points on the UPDRS III scale, was in keeping with other studies,16 although direct comparison is difficult because rate of progression in PD may not be linear.17 Our UPDRS data contrast with the findings of Jankovic and Kapadia, who reported a more rapid decline in UPDRS III in PIGD compared with TD patients, after adjustment for age at initial visit.17 We found no statistically significant difference between rate of UPDRS III progression and motor subtype in the PD subjects in our study, although the absolute deterioration at two years was greater in the PIGD subgroup than in the TD patients (8.9 and 3.6 points, respectively). This disparity may be accounted for by the longer period of follow up in the Jankovic study and the comparatively low numbers of PD subjects in each motor subtype in our study at baseline. Further follow up in our cohort may show a divergence in the rate of motor decline between subtypes. Interestingly, the rate of deterioration in motor function was almost twice as rapid in both of our demented groups (PDD and DLB), compared with PD subjects. We found no significant difference in the rate of either cognitive or motor decline between PD and PDD patients of short compared with long disease duration. The comparatively small numbers in each group and the comparatively short period of follow up necessitate caution in interpreting this finding, however.
Evolution of motor subtype and incident dementia in PD are likely to represent variable involvement of, and rate of neurodegeneration in, diverse neurochemical systems. Increasing cell loss in cholinergic nuclei is likely to underpin cognitive decline in PD,5,18 as well as the emergence of L‐dopa refractory motor features, while recent PET evidence suggests that cell loss in the serotonergic median raphe nucleus may correlate with tremor severity in PD.19 Furthermore, bradykinesia, rigidity, gait, and balance have previously been reported to progress at the same rate in people with PD, while change in tremor was independent of these signs.16
More severe motor symptoms, relating to putative non‐dopaminergic lesions, may be predictive of more rapid cognitive decline,4 and have been associated with incident dementia.6 Our finding, of 25% of the PIGD PD patients developing dementia during the course of this study, compared with no non‐PIGD subtype patients, would support these observations. Differential rates of neurodegeneration within neurochemically diverse brain stem nuclei could also provide a pathophysiological explanation as to why several patients in the PD group evolved from a TD subtype at baseline to indeterminate and PIGD subtypes during follow up.
Strengths of this prospective study include the inclusion of patients diagnosed according to formal diagnostic criteria and verified by consensus agreement, and the use of both CAMCOG and MMSE to record cognitive changes. Furthermore, this is the first study that we are aware of to compare rates of cognitive and motor decline in DLB subjects, as well as PD and PDD groups.
Potential weaknesses include the comparatively high drop out rate during the study and, subsequently, the small patient numbers in some motor subtype groups at follow up. More demented patients were lost to follow up, mainly because of increased mortality. We believe that this is unlikely to have significantly changed the conclusions of the study, however, because such patients would be predicted to have more rapid rates of cognitive and motor decline. Thus, our data for PDD and DLB groups would be, if anything, a conservative estimate. Only five of 40 PD subjects (12.5%) were lost to follow up, so it is improbable that our conclusions relating to motor phenotype and cognitive decline in PD would have been affected. A follow up period of two years is still comparatively short, however, and it must be acknowledged that rate of decline in both cognitive and motor performance is unlikely to be linear over the natural history of Lewy body disease.17,20,21 Furthermore, recruitment of all study groups was from outpatient clinics, while all PD subjects were aged 65 or over, thereby potentially limiting the generalisability of our results.
In conclusion, a PIGD motor subtype of PD is associated with a more rapid rate of cognitive decline, while incident dementia occurs more commonly in this subtype of PD. This information may be useful in informing future trials of putative cognitive neuroprotective agents in PD populations.
Abbreviations
PIGD - postural instability gait difficulty
PD - Parkinson's disease
PDD - Parkinson's disease patients with dementia
DLB - dementia with Lewy bodies
MMSE - mini‐state mental examination
TD - tremor dominant
DSM‐IV - diagnostic and statistical manual
UPDRS - unified Parkinson's disease rating scale
CAMCOG - Cambridge cognitive examination
GDS - geriatric depression scale
Footnotes
Funding: this work was supported by the Medical Research Council (UK). SM was funded by the Healthcare Foundation and LMA by the Alzheimer's Society. The Alzheimer Research Trust supported ENR.
Competing interests: none declared
References
- 1.Aarsland D, Andersen K, Larsen J P.et al Prevalence and characteristics of dementia in Parkinson disease. Arch Neurol 200360387–392. [DOI] [PubMed] [Google Scholar]
- 2.Levy G, Schupf N, Tang M X.et al Combined effect of age and severity on the risk of dementia in Parkinson's disease. Ann Neurol 200251722–729. [DOI] [PubMed] [Google Scholar]
- 3.Foltynie T, Brayne C, Barker R A. The heterogeneity of idiopathic Parkinson's disease. J Neurol 2002249138–145. [DOI] [PubMed] [Google Scholar]
- 4.Aarsland D, Andersen K, Larsen J P.et al The rate of cognitive decline in Parkinson's disease. Arch Neurol 2004611906–1911. [DOI] [PubMed] [Google Scholar]
- 5.Tiraboschi P, Hansen L A, Alford M.et al Cholinergic dysfunction in diseases with Lewy bodies. Neurology 200054407–411. [DOI] [PubMed] [Google Scholar]
- 6.Burn D J, Rowan E N, Minnett T.et al Extrapyramidal features in Parkinson's disease with and without dementia and dementia with Lewy bodies: a cross‐sectional comparative study. Mov Disord 200318884–889. [DOI] [PubMed] [Google Scholar]
- 7.Gibb W R G, Lees A J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 198851745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folstein M F, Folstein S E, McHugh P R. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 197512189–198. [DOI] [PubMed] [Google Scholar]
- 9.McKeith I G, Galasko D, Kosaka K.et al Consensus guidelines for the clinical and pathological diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996471113–1124. [DOI] [PubMed] [Google Scholar]
- 10.Fahn S, Elton R L, and members of the UPDRS development committee Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Goldstein M, et al, eds. Recent developments in Parkinson's disease. New York: Macmillan, 1987153–163.
- 11.Jankovic J, McDermott M, Carter J.et al Variable expression of Parkinson's disease: a base‐line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 1990401529–1534. [DOI] [PubMed] [Google Scholar]
- 12.Hobson P, Meara J. The detection of dementia and cognitive impairment in a community population of elderly people with Parkinson's disease by the use of the CAMCOG neuropsychological test. Age Ageing 19992839–43. [DOI] [PubMed] [Google Scholar]
- 13.Yesavage J A, Brink T L, Rose T L.et al Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 19821737–49. [DOI] [PubMed] [Google Scholar]
- 14.Ballard C G, O'Brien J, Morris C M.et al The progression of cognitive impairment in dementia with Lewy bodies, vascular dementia and Alzheimer's disease. Int J Geriatr Psychiatry 200116499–503. [DOI] [PubMed] [Google Scholar]
- 15.Olichney J M, Galakso D, Salmon D P.et al Cognitive decline is faster in Lewy body variant than in Alzheimer's disease. Neurology 199851351–357. [DOI] [PubMed] [Google Scholar]
- 16.Louis E D, Tang M X, Cote L.et al Progression of parkinsonian signs in Parkinson disease. Arch Neurol 199956334–337. [DOI] [PubMed] [Google Scholar]
- 17.Jankovic J, Kapadia A S. Functional decline in Parkinson's disease. Arch Neurol 2001581611–1615. [DOI] [PubMed] [Google Scholar]
- 18.Bohnen N I, Kaufer D I, Ivanco L S.et al Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease. Arch Neurol 2003601745–1748. [DOI] [PubMed] [Google Scholar]
- 19.Doder M, Rabiner E A, Turjanski N.et al Tremor in Parkinson's disease and serotonergic dysfunction: An 11C‐WAY 100635 PET study. Neurology 200360601–605. [DOI] [PubMed] [Google Scholar]
- 20.Ballard C, O'Brien J, Swann A.et al One year follow‐up of parkinsonism in dementia with Lewy bodies. Dement Geriatr Cogn Disord 200011219–222. [DOI] [PubMed] [Google Scholar]
- 21.Helmes E, Bowler J V, Merskey H.et al Rates of cognitive decline in Alzheimer's disease and dementia with Lewy bodies. Dement Geriat Cog Disord 20031567–71. [DOI] [PubMed] [Google Scholar]