Abstract
Clinical and laboratory characteristics of 39 patients with adult onset subacute sclerosing panencephalitis (SSPE) are described and compared to those of juvenile onset patients regarding preceding measles, age at onset, gender, interval between onset and diagnosis, clinical profile, and course during follow up. Diagnosis was based on clinical and electroencephalographic findings and raised anti‐measles antibody titres in cerebrospinal fluid. Mean age at SSPE symptom onset was 20.9±4.9 years and mean interval from onset to diagnosis was 6.3±9.6 months. Referral diagnosis was accurate in only 12 patients. Presenting symptoms included myoclonus, behavioural changes, seizures, and cognitive, visual, and extrapyramidal disturbance. All patients received symptomatic therapy; 19 also received disease modifying agents. Five of seven pregnant women had successful deliveries. The follow‐up period varied widely (maximum 60 months, median 9 months). The profile of adult onset SSPE did not differ from the rest of the cohort, except for a longer interval between measles infection and symptom onset (p<0.0001). SSPE in adults poses diagnostic challenges for clinicians. A high index of suspicion and appropriate investigations are necessary for early diagnosis and counselling.
Keywords: adult onset, measles, SSPE, subacute sclerosing panencephalitis
Subacute sclerosing panencephalitis (SSPE) is attributed to infection by mutated measles virus and is characterised by progressive mental deterioration, motor decline, and myoclonus leading to death within 1–3 years of onset. SSPE is essentially a disease of childhood and commonly occurs at 5–15 years of age. The introduction of measles vaccination under mass immunisation programs has led to a significant fall in the incidence of measles and SSPE throughout the world. Although the mean age at onset of SSPE has been reported to be increasing,1 the literature regarding adult onset SSPE remains rather sparse.2,3,4,5,6,7,8 This report, therefore, describes various demographic, clinical, and laboratory features of adult onset (over 18 years of age) SSPE patients.
Methods
This retrospective study was conducted at the National Institute of Mental Health and Neuro Sciences (NIMHANS), Bangalore, a tertiary care university teaching hospital in South India and involved 307 SSPE patients evaluated during the period January 1995 to December 2004. Diagnosis was based on the characteristic clinical features of progressive cognitive and/or behavioural changes, myoclonus, electroencephalographic evidence of periodic complexes, and raised IgG (⩾1:25) anti‐measles antibody in cerebrospinal fluid (CSF), detected by ELISA in all patients. This cut‐off CSF titre was established in our neurovirology laboratory after evaluation in 1986–87 of over 300 CSF samples obtained from patients with neurological illnesses other than SSPE (brain tumours, head injuries, other CNS infections) and individuals undergoing spinal anaesthesia for minor surgical procedures.9 Analysis for oligoclonal bands in CSF was not carried out. We have not performed any specific test to determine disruption of the blood‐brain barrier; however, during the 1980s and 1990s we carried out oligoclonal antibody banding in some patients which showed that anti‐measles antibodies in the CSF were indeed due to intrathecal production and not caused by passive transfer of antibodies as a result of disruption of the blood‐brain barrier.10
Thirty nine patients aged 18 years and above at first symptom onset were considered to have adult onset SSPE and their data regarding measles vaccination, any history of measles, age at onset of symptoms and diagnosis, presenting symptoms, initial diagnosis, clinical staging, course of the disease, electroencephalographic changes, and measles antibody status were recorded.
SPSS v10 software was used for statistical analysis. Student's t test and Pearson χ2 test were applied to compare various parameters. Data were considered significant for p values less than 0.05 (p⩽0.05).
Results
Demographic profile
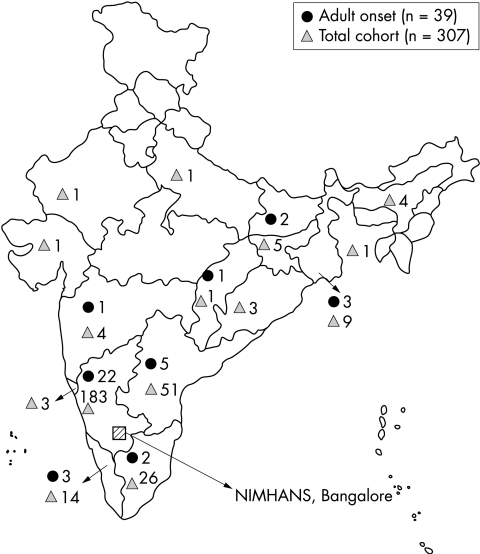
Most (82%) patients (n = 307) in this cohort were from South India (fig 1), as were most patients with adult onset SSPE (n = 32). However, a few patients were from eastern India (n = 5) and other parts of the country (n = 2) (fig 1). Of these 307 patients, 39 (12.7%; 25 males, 14 females) experienced the first symptoms of SSPE after 18 years of age, the mean age at onset of symptoms being 20.9±4.9 years (range: 18–43 years). Mean age at diagnosis was 21.3±5.1 years and ranged widely from 18 to 43 years. The interval between the onset of symptoms and diagnosis was 6.3±9.6 months (range: 0.3–60 months). A history of measles was available for 13 of 34 patients where the information was recorded. Symptoms of SSPE were noted at a mean interval of 16.7±6.3 years (range: 8–33 years) following measles infection.
Figure 1 Map showing the demographic profile of adult onset SSPE patients and the entire cohort of SSPE patients.
Clinical profile
The referral diagnosis was SSPE in 12 patients, while in others it varied widely and included psychosis, complex partial seizures, viral retinitis, rheumatic chorea, seizure disorder, vasculitis, neurometabolic syndrome, extrapyramidal syndrome, and juvenile myoclonic epilepsy. The mean interval between the first symptom and evaluation at our centre was much longer (13.1±25.4 months) in patients with an inaccurate diagnosis compared to patients with the correct diagnosis (4.8±4.9 months, p = 0.27).
The presenting manifestations were myoclonus (25), behavioural changes (five), seizures (three), cognitive impairment (two), visual disturbances (two), and extrapyramidal symptoms (two). Myoclonus characteristically was slow and was the initial feature in 64.1% of patients. However, during the course of the disease, all patients developed myoclonus involving limb and axial musculature. Myoclonus was asymmetrical in 11 patients (28.2%). Eighteen patients had gait abnormality and falls probably due to myoclonus. The behavioural and cognitive symptoms consisted of poor academic performance, forgetfulness, recent change in personality, dullness, and apathy. Seven patients had pyramidal weakness (four on the right and three on the left). Two patients presented with impaired vision. However, during the course of the disease, two additional patients also developed visual symptoms. One patient was blind from birth due to bilateral micro cornea and coloboma. A total of nine patients had seizures during the course of the illness (two partial and seven secondary generalised), in three of whom it was the initial manifestation. No patient had status epilepticus. Tremors were reported at onset in two patients and appendicular dystonia was subsequently noted in three patients.
Seven of the 14 women were pregnant. Four patients had onset of symptoms during pregnancy and one patient had worsening of symptoms during pregnancy. Two patients conceived after the diagnosis of SSPE was established. Of these seven patients, five had a successful delivery; details regarding the outcome of delivery were not available in the other two patients.
Imaging
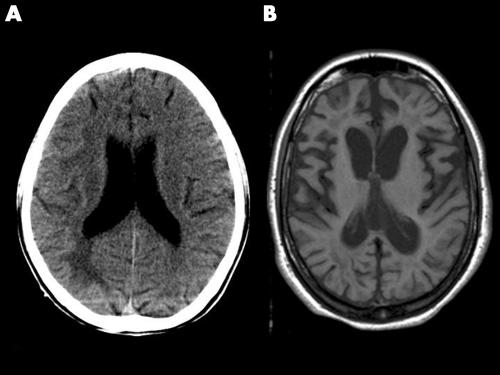
Imaging studies were carried out at some time during the course of the illness in all but two patients (computed tomography (CT) in 26 and magnetic resonance imaging (MRI) in 12). The interval between the onset of symptoms and CT imaging ranged from 1 month to 4 years (mean: 5.5±9.2 months). Fourteen patients had normal CT conducted between 1 month and 1 year after symptom onset. In others, the CT changes were as follows: white matter hypodensities involving frontal/parietal/occipital/temporal regions (two), cerebral atrophy (six), thalamic hypodensities (one), and diffuse oedema (two) (fig 2A). MRI was carried out in 12 patients between 1 month and 4 years (mean: 9.3±13.2 months) after the onset of symptoms. Seven patients had normal MRI conducted between 2 and 18 months after the onset of symptoms. In the remaining five patients, the MRI abnormalities included focal or diffuse white matter signal changes in three patients and cerebral atrophy of varying degrees in the other two (fig 2B).
Figure 2 (A) Non‐contrast axial section of computed tomography brain image showing white matter hypodensity in a 20 year old patient 1 month after symptom onset. (B) Axial section of T1W magnetic resonance image of a 22 year old patient showing diffuse atrophy 48 months after symptom onset.
Laboratory studies
All patients underwent CSF analysis at NIMHANS at a mean interval of 7.1±10.2 months (range: 2 weeks to 60 months) after the first symptom. The results were as follows: glucose 58.8±10.5 mg/dl (range: 38–78 mg/dl), protein 39.6±21.9 mg/dl (range: 10–96 mg/dl), and cells 1.9±2.7 cells/mm3 (range: 0–9 cells/mm3). Fourteen patients had raised CSF protein levels of >45 mg/dl and four patients had pleocytosis (>5 cells/mm3). ELISA was used for estimation of anti‐measles antibody and the diagnostic titre of ⩾1:25 was observed in all patients. Simultaneous estimation of anti‐measles antibody in the serum was carried out in 22/39 patients. The ratios of serum to CSF anti‐measles antibody in these patients ranged from 1 to 10.
Follow up
Nineteen patients were treated with disease modifying agents for variable periods of time: isoprinosine (12), intravenous immunoglobulin (two), oral steroids (six), levamisole (five), amantadine (seven), methylprednisolone (one), and plasmapheresis (one). All patients also received symptomatic treatment for myoclonus and seizures. The follow‐up period from the onset of symptoms was variable: 1 year or less in 27 patients; 1–2 years in eight, 2–3 years in two, and 38 months and 60 months in one patient each (mean: 11.1±11.4 months). There was no subjective difference in the outcome between untreated and treated groups. However, evaluation did not include any objective measures/scales.
Comparison with childhood onset SSPE
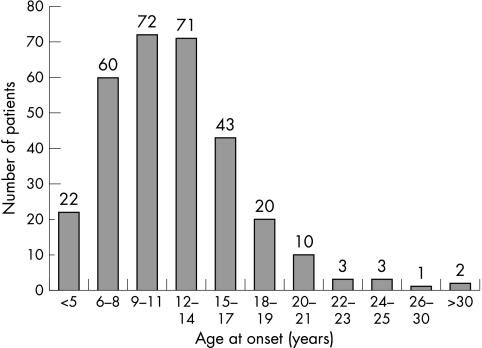
The data on adult onset SSPE were compared with that of the rest of the cohort as regards age at onset, interval from onset to diagnosis, gender distribution, interval between measles infection and onset of symptoms, clinical symptoms, and duration of follow up. There was no significant difference in any of these parameters between the two groups, except that the interval between measles infection and onset of symptoms on Student's t test (p<0.0001) was longer in the adult onset group (table 1). Onset of symptoms among all evaluated SSPE patients showed peak incidence between 9 and 14 years of age. In the adult onset cases, most patients (n = 28) developed symptoms at or before 20 years of age (fig 3).
Table 1 Comparison between adult onset and paediatric onset SSPE.
| Parameter | Adult onset (⩾18 years) | Paediatric onset (<18 years) | p |
|---|---|---|---|
| Number of patients | 39 | 268 | – |
| Male/female | 25/14 | 202/66 | 0.11 |
| Age at onset, years | 20.9±4.9 | 10.5±3.6 | 0.0001* |
| Duration from onset to | 6.3±9.6 | 5.6±8.5 | 0.68 |
| diagnosis, months | |||
| Interval between measles | 16.7±6.3 | 7.4±3.5 | 0.0001* |
| and symptoms, years | (n = 13) | (n = 96) | |
| Myoclonus | 39 | 249 | 0.1 |
| Lateralising deficits | 7 | 53 | 0.33 |
| Visual disturbances | 5 | 23 | 0.4 |
| Seizures | 9 | 93 | 0.14 |
| Follow‐up duration, | 11.1±11.4 | 12.9±23.8 | 0.64 |
| months |
*Significantly correlated on Student's t test.
Figure 3 Bar diagram showing age distribution of patients with SSPE.
Discussion
Subacute sclerosing panencephalitis is a prototype neurodegenerative disease of childhood characterised by onset of neurological symptoms between 5 and 15 years of age with a progressive downhill course leading to death within 2–4 years.1 The incidence of adult onset SSPE has been reported to vary between 1–1.75% and 2.6%.11,12 To date less than 100 cases of adult onset SSPE have been published in the literature, the majority being case reports2,3,6,7,8,13,14 baring one exception.4 The present series of 39 patients constituted 12.7% of the cohort evaluated over a decade at our centre. To the best of our knowledge this is the largest series of adult onset SSPE patients. Most of our patients were from South India; whether a similar incidence of adult onset cases of SSPE is observed elsewhere in India is not known.
Tan et al4 reported eight cases of adult onset SSPE (⩾18 years) seen between 1980 and 1987 at a university hospital in Turkey. Age at onset was between 18 and 24 years, with a 1–9 month interval between first symptoms and diagnosis. In the published literature on adult onset SSPE, the age at onset was 20–35 years with a mean delay in diagnosis of 10.6 months.5 A similar time lag of 6.3±9.6 months in diagnosis was observed in the present study. In childhood onset SSPE, boys are more frequently affected,1 while in adults the gender distribution is reported to be equal.4,5 However, in our series there was a preponderance of males (25 males, 14 females) (table 2).
Table 2 Comparison between various published adult onset SSPE case series.
| Parameter | Tan et al4 | Singer et al5 | Present series |
|---|---|---|---|
| Number of cases | 8 | 13 | 39 |
| Study period | 1980–1987 | 1965–1997 | 1995–2004 |
| Design | Retrospective | Case reviews | Retrospective |
| Mean age, years | 19.6 | 25.4 | 20.9 |
| (range) | (18–24) | (20–35) | (18–43) |
| Gender (M/F) | 4/4 | 7/6 | 25/14 |
| History of measles | 5 | 5 | 13 |
| Duration of symptoms | 1–9 | NA | 6.3±9.6 |
| before diagnosis, | |||
| months | |||
| Follow‐up duration, | 7–38 | 8–32 | 1–60 |
| months |
Singer et al5 have suggested that adult onset SSPE patients get measles either at a very early age (<3 years) or at an unusually late age (>9 years). However, this was not noted either by us or by Tan et al.4 A history of prior infection of measles in childhood was available in 13 patients in our series and the interval of 16.7±6.3 years between measles infection and the onset of neurological symptoms was significantly longer compared with paediatric onset SSPE (p<0.0001). The age at measles infection among these patients was 1 year (two), 3 years (two), 4 years (one), 5 years (four), and 10 years (two).
Certain differences have been recorded between adult onset and childhood onset SSPE. Singer et al5 observed that only two of 13 adult onset patients presented with personality change, while eight had purely ophthalmic manifestations. In the current series, 32 patients presented with either cognitive and behavioural changes or myoclonus and only two had ophthalmic symptoms at onset.
Tan et al4 noted that two out of four women had their first manifestations of SSPE during pregnancy. In the present series, four out of seven women who conceived had onset of symptoms during the course of pregnancy or during puerperium. During pregnancy specific and innate immune systems are altered for adaptation to foreign antigens of the fetus and placenta. The immunosuppressant agents or cells produced by the fetus, such as fetal suppressor T cells, alpha‐fetoprotein, and lymphokines, enter the maternal circulation and modify cell mediated immunity. This suppression of cell mediated immunity may lead to more severe virus infection either in the mother or in the fetus. Influenza, hepatitis E, polioviruses, and chicken pox are associated with increased severity of maternal illness during pregnancy, whereas rubella, parvovirus, cytomegalovirus, herpes simplex, and human immunodeficiency virus are associated with increased fetal damage.15 However, whether pregnancy precipitates or modifies the natural course of SSPE remains to be established.
The course of adult onset SSPE is not clearly known due to the paucity of reports in the literature. It is generally postulated that SSPE has an aggressive course in adults.13,16 Tan et al4 reported that all but one of seven patients died within 1 year of symptom onset. They proposed that survival of patients with SSPE becomes shorter with increasing age at onset. However, Singer et al5 observed a mean survival period of 24 months (range: 8 months to 6 years) with three patients surviving for more than 3 years. They believe that patients in the adult onset group have a higher rate of spontaneous remission and favourable response to disease modifying agents. In the present series, the median duration of follow up was only 9 months and therefore it is not possible to comment on the length of patient survival.
Conclusions
What governs the interval between the occurrence of measles and the onset of SSPE? Whether the low virulence of mutated measles virus or the immunological status of the host accounts for late occurrence needs to be explored. This retrospective study and observations by others suggest that SSPE occurs in adults and may follow a much earlier measles infection. A high index of suspicion may be required during initial stages of the disease for early diagnosis, proper counselling, and therapeutic intervention. Descriptions of similar cases, long term follow up, and research into the agent and host factors in adult onset SSPE may improve our knowledge regarding the pathophysiology of this preventable disease.
Footnotes
Competing interests: none declared
References
- 1.Dyken P R. Subacute sclerosing panencephalitis. Current status. Neurol Clin 19853179–196. [PubMed] [Google Scholar]
- 2.Nelson R F, Dennery J M, Montpetit V.et al SSPE and pregnancy. Lancet 197211289. [DOI] [PubMed] [Google Scholar]
- 3.Cape C A, Martinez A J, Robertson J T.et al Adult onset of subacute sclerosing panencephalitis. Arch Neurol 197328124–127. [DOI] [PubMed] [Google Scholar]
- 4.Tan E, Namer I J, Ciger A.et al The prognosis of subacute sclerosing panencephalitis in adults. Report of 8 cases and review of the literature. Clin Neurol Neurosurg 199193205–209. [DOI] [PubMed] [Google Scholar]
- 5.Singer C, Lang A E, Suchowersky O. Adult onset subacute sclerosing panencephalitis: case reports and review of the literature. Mov Disord 199712342–353. [DOI] [PubMed] [Google Scholar]
- 6.Vela L, Garcia‐Merina A, Escamilla C. Adult onset subacute sclerosing panencephalitis first seen as craniocervical myoclonus. Mov Disord 199712462–464. [DOI] [PubMed] [Google Scholar]
- 7.Frings M, Blaeser I, Kastrup O. Adult onset subacute sclerosing panencephalitis presenting as a degenerative dementia syndrome. J Neurol 2002249942–943. [DOI] [PubMed] [Google Scholar]
- 8.Mawrin C, Lins H, Koenig B.et al Spatial and temporal disease progression of adult onset subacute sclerosing panencephalitis. Neurology 2002581568–1571. [DOI] [PubMed] [Google Scholar]
- 9.Poornima K S, Ravi V, Desai A S.et al A sero‐epidemiological study in south India. In: Pant B, Prabhakar S, eds. Proceedings of the Third International Symposium on SSPE. Vellore: Christian Medical College, 1989173–175.
- 10.Shetty K T, Yogen T H, Ravi V.et al Isoelectrofocussing profile of CSF proteins in subacute sclerosing panencephalitis. In: Pant B, Prabhakar S, eds. Proceedings of the Third International Symposium on SSPE. Vellore: Christian Medical College, 1989145–149.
- 11.Haddad F S, Risk W S, Jabbour J T. Subacute sclerosing panencephalitis in the Middle East: report of 99 cases. Ann Neurol 19771211–217. [DOI] [PubMed] [Google Scholar]
- 12.Yalaz K, Anlar B, Renda Y.et al Subacute sclerosing panencephalitis in Turkey: epidemiologic features. 1st meeting of the Association of Mediterranean Child Neurology. Crete, Greece: June 8–11, 1987
- 13.Nagaraja D, Shankar S K, Krishna Murthy L.et al Subacute sclerosing panencephalitis – a clinical and pathological study. NIMHANS J 19853101–108. [Google Scholar]
- 14.Scully R E, Mark E J, McNeely W F.et al Case records of the Massachusetts General Hospital. N Engl J Med 19983381448–1456.9583972 [Google Scholar]
- 15.Johnson R T. Infections. In: Hainline B, Devinsky O, eds. Advances in neurology. Vol 90. Neurological complications of pregnancy. Philadelphia, PA: Lippincott, Williams and Wilkins, 2002145–156.
- 16.Garg R K. Subacute sclerosing panencephalitis. Postgrad Med J 20027863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]