Abstract
Background
Antipsychotic treatment in schizophrenia is frequently associated with extrapyramidal side effects. Objective behavioural measures to evaluate the severity of extrapyramidal side effects in the clinical setting do not exist.
Objectives
This study was designed to investigate grasping movements in five drug naive and 13 medicated subjects with schizophrenia and to compare their performance with that of 18 healthy control subjects. Deficits of grip force performance were correlated with clinical scores of both parkinson‐like motor disability and psychiatric symptom severity
Methods
Participants performed vertical arm movements with a handheld instrumented object and caught a weight that was dropped into a handheld cup either expectedly from the opposite hand or unexpectedly from the experimenter's hand. The scaling of grip force and the temporospatial coupling between grip and load force profiles was analysed. The psychiatric symptom severity was assessed by the positive and negative symptom score of schizophrenia and the brief psychiatric rating scale. Extrapyramidal symptoms were assessed by the unified Parkinson's disease rating scale.
Results
Drug naive subjects with schizophrenia performed similar to healthy controls. In contrast, medicated subjects with schizophrenia exhibited excessive grip force scaling and impaired coupling between grip and load force profiles. These performance deficits were strongly correlated with the severity of both extrapyramidal side effects related to antipsychotic therapy and negative symptoms related to the underlying pathology.
Conclusions
These data provide preliminary evidence that deficits of sensorimotor performance in schizophrenia are, at least in part, related to the side effects of antipsychotic treatment. The investigation of grasping movements may provide a sensitive measure to objectively evaluate extrapyramidal side effects related to antipsychotic therapy.
Keywords: grasping, predictive force control, neuroleptics, side effects, reactive force control
Almost a century ago, Bleuler1 and Kraepelin2 described several motor deficits in schizophrenia, such as dyscoordination of hand and arm movements when performing crafts. In this era antipsychotic drugs did not exist and, consequently, these early clinical findings cannot be considered a side effect of drug treatment. Today, antipsychotic treatment is widely used and frequently associated with basal ganglia dysfunction causing parkinson‐like symptoms, such as tremor, increased muscle stiffness, reduced movement speed, and postural imbalance.3,4 Surprisingly, most current studies investigating motor performance in schizophrenia seem to simply neglect the possibility of antipsychotic side effects or struggle with the problems associated with the recruitment of drug naive subjects.5,6,7 Only a few studies investigated motor performance in drug free and medicated subjects with schizophrenia.8,9 The motor disability in schizophrenia is currently held to be a problem in predictive control of movement.6,7,10 However, the exact nature and cause of the motor disability in schizophrenia still remains to be elucidated.
The combined role of motor concepts for predictive and reactive force control is well established in grasping.11,12,13,14,15 When we transport environmental objects the applied grip force is always adjusted to be slightly higher than the minimum required to prevent the object slipping against the loads induced by movement.11,15 Grip force is modulated in parallel with load without an obvious time delay, suggesting that the central nervous system can predict the load variations induced by voluntary movements before their occurrence.11,13 If, however, the force acting on the object is unexpectedly changed, for example, by dropping a mass into a handheld cup, then the adjustment of grip force lags behind the increase in load, showing a switch from predictive to reactive control.14 In contrast, if the subject can estimate the time of impact, for example by dropping the weight from one hand into a cup held by the opposite hand, then grip force is adjusted in a predictive manner before impact.14
This study was designed to answer the question of how parkinson‐like side effects, induced by antipsychotic treatment, affect predictive and reactive mechanisms of grip force control. Grip force coordination when transporting a handheld load and when catching a weight was assessed in medicated and drug naive subjects with schizophrenia. The performance of subjects with schizophrenia was compared with that of healthy subjects and performance deficits were correlated with clinical scores of both psychiatric symptom severity and motor disability.
Methods
Participants
Eighteen right handed subjects with schizophrenia (nine women; mean age = 36 years, SD = 11 years) and 18 right handed healthy sex and age matched control subjects (nine women; mean age = 36 years, SD = 12 years) participated in the study. Exclusion criteria for were a history of neurological disease or trauma, alcohol or other substance misuse, and age younger than 18 years. All participants gave written informed consent. The study was approved by the local ethics committee.
Psychiatric diagnosis of each subject with schizophrenia was established in accordance with the DSM‐IV‐TR criteria for schizophrenia. The average disease duration was 10 years (SD = 8 years, range = 0.5–30 years) and the average number of admissions to hospital was seven (SD = 7, range = 1–26). The average level of symptom severity was rated with the brief psychiatric rating scale (total score: 41, SD = 14, range = 18–70).16 The average scores on the scale for the assessment of positive and negative symptoms17 were 10.4 (SD = 5.8, range = 3–22) for positive symptoms and 15.4 (SD = 6.1, range = 8–25) for negative symptoms. Thirteen subjects with schizophrenia were receiving antipsychotic drugs. Dose was stable for at least one week until and during testing. Five subjects with schizophrenia had never received antipsychotic drugs. None of the subjects with schizophrenia received antiparkinsonian drugs. The average motor subscore (subitems 18–31) of the unified Parkinson's disease rating scale (UPDRS),18 used to rate the patients' motor disability associated with antipsychotic treatment, was 14.7 (SD = 13, range = 0–40). Table 1 summarises the demographic and clinical data.
Table 1 Demographic and clinical data of subjects with schizophrenia.
| Patient | Sex, age in years | Symptom duration in years | Number of hospital stays | BPRS score | PANSS score positive symptoms | Negative symptoms | Motor subscore of the UPDRS | Antipsychotic treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | F, 22 | 1 | 3 | 44 | 20 | 10 | 0 | none |
| 2 | F, 31 | 1 | 1 | 37 | 12 | 18 | 0 | none |
| 3 | F, 31 | 0.5 | 2 | 44 | 7 | 23 | 0 | none |
| 4 | M, 22 | 0.5 | 1 | 57 | 8 | 17 | 0 | none |
| 5 | M, 34 | 0.5 | 2 | 46 | 19 | 12 | 0 | none |
| 6 | F, 55 | 6 | 6 | 52 | 8 | 12 | 30 | risperidone 37.5 mg intramuscularly (gluteal muscle) every 2 weeks |
| 7 | F, 33 | 16 | 15 | 18 | 3 | 24 | 52 | ziprasidone 80 mg orally/day |
| 8 | M, 33 | 6 | 5 | 45 | 8 | 18 | 17 | amisulpride 200 mg orally/day; olanzapine 10 mg orally/day |
| 9 | M, 40 | 30 | 26 | 61 | 13 | 25 | 33 | ziprasidone 80 mg orally/day |
| 10 | F, 58 | 20 | 17 | 30 | 3 | 20 | 44 | amisulpride 600 mg orally/day |
| 11 | M, 28 | 0.5 | 1 | 50 | 13 | 11 | 8 | olanzapine 20 mg orally/day |
| 12 | F, 44 | 15 | 12 | 31 | 9 | 9 | 12 | ziprasidone 160 mg orally/day |
| 13 | F, 42 | 5 | 8 | 30 | 5 | 9 | 15 | amisulpride 500 mg orally/day |
| 14 | M, 28 | 2 | 4 | 24 | 6 | 8 | 8 | amisulpride 400 mg orally/day |
| 15 | M, 22 | 6 | 8 | 30 | 8 | 9 | 10 | risperidone 2 mg orally/day; clopenthixole 160 mg orally/day |
| 16 | M, 33 | 10 | 3 | 70 | 17 | 22 | 33 | risperidone 6 mg orally/day |
| 17 | F, 54 | 10 | 4 | 34 | 6 | 9 | 22 | ziprasidone 160 mg orally/day |
| 18 | M, 36 | 12 | 10 | 36 | 22 | 21 | 40 | olanzapine 20 mg orally/day |
M, male; F, female; BPRS, brief psychiatric rating scale; PANSS, positive and negative syndrome scale; UPDRS, unified Parkinson's disease rating scale (motor subscore = items 18–31).
Instrumented object
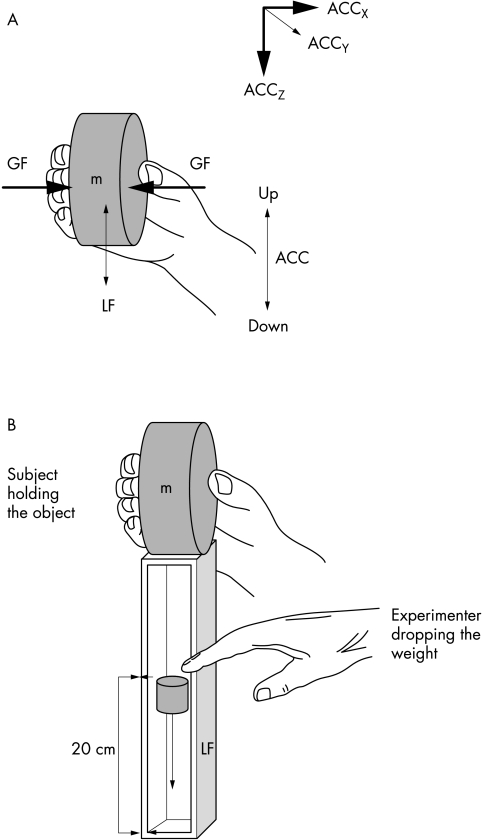
Participants grasped a cylindrical and cordless instrumented object with the dominant right hand (fig 1). Technical details about the instrumented object were provided elsewhere.12 The object incorporated a force sensor for grip force registration and linear acceleration sensors for registration of acceleration signals in three dimensions. Grip surfaces were sandpaper at a medium grain (number 240) in all trials performed. For arm movement experiments the object was used alone (mass 0.35 kg). For the weight catching experiments the object was mounted onto a cup (mass 0.37 kg) in which a 100 g weight was dropped.
Figure 1 Configuration of the hand and fingers applied when performing vertical arm movements with the handheld object (A) and performing the weight catching task in the experimenter release condition (B). GF, grip force; LF, load force; ACC, linear acceleration.
Experimental procedures
Before the experiments participants washed their hands with water and soap and carefully dried them. The experimenter gave verbal instructions, demonstrated the procedure, and observed the participants as they did the tasks.
Vertical arm movements
Participants were instructed to move the object fast between two points 30 cm apart on a straight, vertical line and to keep its orientation constant. Short breaks of about one second were introduced in between single up and down movements. Five trials consisting of 10 movements with inter‐trial breaks of 30–60 seconds were performed. After the experiment, participants were asked to slowly release the object until it dropped to the support. This procedure was repeated twice to obtain an estimate of the minimal grip force necessary to prevent the object from slipping. The slip point was defined as the first detectable change in acceleration along the object's vertical Z axis and the minimum grip force was determined at this time point.
Weight catching task
Participants sat in a stable chair with the dominant arm slightly abducted, the elbow resting on the right thigh and the forearm held unsupported and rotated in front to the trunk with the elbow flexed at about 90°. Participants held the object mounted onto a cup (fig 1B). Participants were instructed to hold the object stationary and to prevent it from slipping. In the experimenter release condition, participants were asked to keep their eyes closed for the entire experiment. The experimenter dropped a 100 g weight unexpectedly into the cup from a height of 20 cm (fig 1B). In the self release experiment, participants themselves dropped the 100 g weight into the cup with their eyes open. Ten such trials with inter‐trial intervals of five seconds were performed for the experimenter and self release conditions, respectively.
Data analysis and statistics
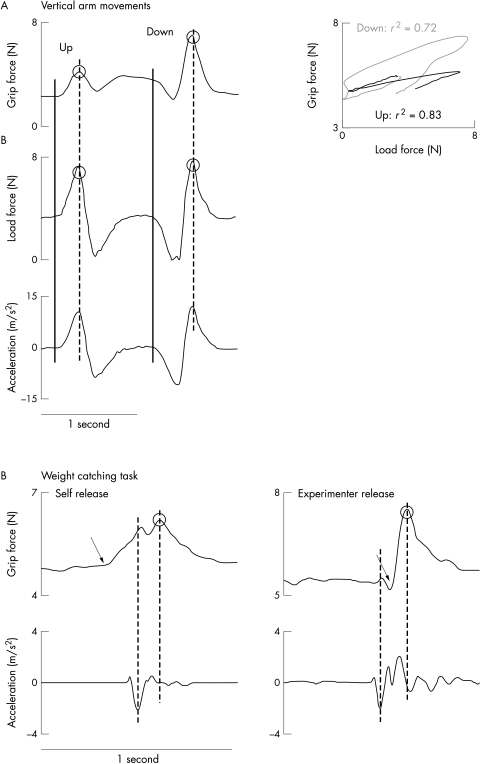
Positive kinematic acceleration (ACC) of the object was directed upward. The net load force (LF) was calculated from the object mass and the vectorial summation of gravity (9.81 m/s2) and inertial accelerations along the object's X, Y, and Z axes. Figure 2 shows the parameters obtained for data analysis.
Figure 2 Illustration of the parameters obtained for data analysis from the arm movement task (A) and weight catching task (B) in the self release and experimenter release conditions. The performance of a healthy control subject (woman, 31 years) is illustrated. (A) Circles indicate peak grip and load forces. The closed vertical line indicates movement onset, the dotted vertical line shows peak acceleration. The panel to the right represents plots of grip force compared with load force for the course of upward and downward movements. The correlation coefficients of the least square regression lines are indicated for both movements. (B) Circles indicate peak load forces. The dotted vertical lines show the time of peak downward acceleration attributable to impact and peak grip force. The arrows refer to the onset of grip force increase. Note the onset of grip force occurred close to peak downward acceleration for the weight catching trial in the self release condition, but after peak downward acceleration in the experimenter release condition.
Vertical arm movements
To exclude learning effects, the first trial was excluded from the data analysis. Two time points within the course of voluntary movements were determined (fig 2A): (1) movement onset as determined when the acceleration signal deviated more than two standard deviations from the baseline level and (2) peak acceleration. At these time points grip force and acceleration signals, as well as calculated load force were determined. The ratio between grip and load forces at movement onset and at the time of peak acceleration was calculated. A correlation analysis between grip and load forces was performed for the entire course of each movement to describe the temporospatial coupling between both forces. Squared correlation coefficients (r2) were calculated. Statistically, for each movement direction (up or down) we tested whether the ratio between grip and load forces differed between subjects with schizophrenia and healthy controls, using repeated measures analysis of variance with the between subject factor “group” (subjects with schizophrenia compared with controls) and the within subject factor “direction” (up compared with down). A p value of ⩽0.05 was considered significant. The temporal coupling between grip and load forces (assessed by the squared correlation coefficient) was compared between subjects with schizophrenia and healthy controls using the Mann‐Whitney U test.
Weight catching task
The load perturbation was assessed by determining the peak downward acceleration of the cup attributable to impact. Peak grip force was obtained from the last seven trials in the experimenter and self release conditions (fig 2B). To test the hypothesis that predictive and reactive control mechanisms were maintained in subjects with schizophrenia, regression analyses were performed on the grip force traces obtained from 0.5 second periods starting at the time of peak acceleration. Grip force traces obtained from subjects with schizophrenia (with/without drugs) and controls and from both experimental conditions were correlated. We used repeated measures analysis of variance to investigate the influence of the between subject factor “group” (subjects with schizophrenia compared with controls) and the within subject factor “condition” (experimenter compared with self release) on the peak grip forces. A p value of ⩽0.05 was considered significant. Clinical scores and grip force parameters obtained from both tasks were compared using Spearman rank correlations and significance was assumed if the p value was less than 0.05.
Results
The slip forces were similar (p>0.1 for each comparison) for healthy subjects (2.1 (SD 0.2) N), drug naive subjects with schizophrenia (2.2 (SD 0.2) N) and subjects with schizophrenia receiving medication (2.1 (SD 0.3) N). Consequently, any group differences in scaling of grip forces are unlikely to result from variations in the frictional condition at the skin‐object interface.
Qualitative description
Figure 2 illustrates representative data obtained from the performance of a healthy subject during each task. Grip force was modulated in parallel with load force during vertical arm movements (fig 2A). In the self release condition of the weight catching trial (fig 2B), grip force started to rise before the load perturbation induced by impact, indicating anticipation of the time of impact. When, however, the weight was dropped unexpectedly from the experimenter's hand, grip force lagged behind the time of impact, suggesting reactive force control.
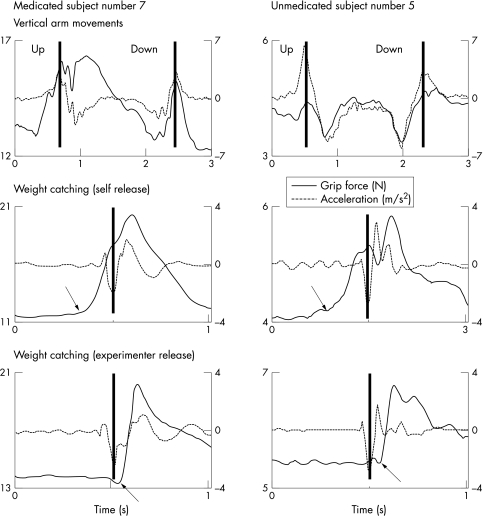
Figure 3 illustrates representative data obtained from trials of two subjects with schizophrenia. Subject 7 did receive antipsychotic treatment, while subject 5 had never received antipsychotic drugs. Subject 5 performed similar to healthy controls. Compared with healthy controls, subject 7 produced greater grip forces, regardless of the task performed, and smaller arm accelerations when transporting the object. The force traces generated by subject 7 also appear to be more irregular. Obviously, antipsychotic drugs result in an overflow of grip force and bradykinesia of voluntary movement. The clinical scores of motor disability confirmed these findings: the motor subscore of the UPDRS was 52 points for subject 7, but zero points for subject 5.
Figure 3 Grip force and acceleration traces obtained from single vertical arm movements with the handheld object and weight catching tasks in the self release and experimenter release conditions performed by subjects with schizophrenia. Subject 7 was receiving antipsychotic treatment, while subject 5 had never received any antipsychotic drugs. The closed vertical lines show peak acceleration. The arrows show the time of grip force increase in the weight catching trials.
Vertical arm movements
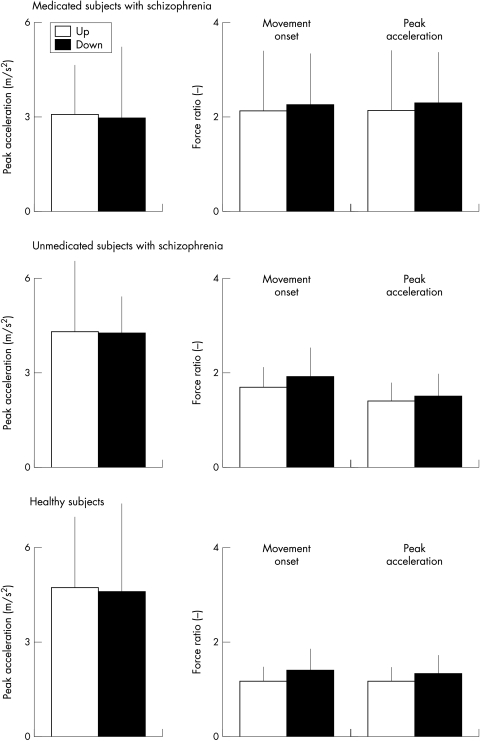
Figure 4 illustrates means of peak accelerations and the ratios between grip and load forces at the time of movement onset and peak acceleration obtained from vertical arm movements within each group. Medicated subjects with schizophrenia exhibited lower peak accelerations than healthy controls (F1,24 = 7.9; p<0.01). Movement direction had no significant effect on the peak accelerations and there was no significant interaction ”group” × “direction”. Peak accelerations were of similar magnitude when comparing healthy subjects with drug naive subjects with schizophrenia, regardless of movement direction. The interaction “group” × “direction” was not significant.
Figure 4 Average values (1 SD) of peak accelerations and the ratio between grip and load forces at the time of movement onset and peak acceleration obtained from vertical arm movements performed by medicated and unmedicated subjects with schizophrenia and healthy controls.
The force ratios at the time of movement onset (F1,24 = 7.8; p<0.01) and at the time of peak acceleration (F1,24 = 5.7; p = 0.02) were greater for subjects with schizophrenia compared with healthy controls. There was no significant effect of movement direction. The interaction “group” × “direction” was not significant. Drug naive subjects with schizophrenia also generated greater force ratios at the time of movement onset (F1,8 = 6.5; p = 0.03), however, we found no significant difference between both groups for the force ratios at the time of peak acceleration. The factor “direction” and the interaction “group” × “direction” were not significant.
The average r2 correlation coefficients (SD) obtained from regression analyses between grip and load force profiles were 0.77 (0.1) for upward movements and 0.80 (0.1) for downward movements of healthy subjects. The correlation coefficients of medicated subjects with schizophrenia were smaller (p⩽0.001) than those of healthy subjects (0.36 (0.2) for upward and 0.48 (0.2) for downward movements). In contrast, the correlation coefficients of drug naive subjects with schizophrenia (0.72 (0.2) for upward and 0.71 (0.1) for downward movements) and healthy subjects were just not significantly different (p⩾0.06). None of the interactions were significant.
When correlating the clinical scores with the behavioural measures of subjects with schizophrenia under antipsychotic treatment we found a significant correlation (p<0.01 for all comparisons) between the force ratio at the time of movement onset and both the UPDRS motor subscore and the negative symptom score of the PANSS for both upward (UPDRS: 0.82; PANSS: 0.65) and downward movements (UPDRS: 0.75, PANSS: 0.72). There was also a significant correlation (p<0.01 for all comparisons) between the force ratios of medicated subjects at the time of peak acceleration and both the UPDRS motor subscore (upward movements: 0.75; downward movements: 0.70) and the negative symptom score of the PANSS (upward movements: 0.65; downward movements: 0.71). There was a significant (p<0.001) correlation between the UPDRS motor score and the negative symptom score for medicated subjects with schizophrenia (0.81). The force ratios were not significantly correlated with the BPRS or the positive symptom score of the PANSS.
There was a significant negative correlation (p<0.01 for all comparisons) between the r2 correlation coefficients of medicated subjects and both the UPDRS motor subscore (upward movements: −0.85; downward movements: −0.89) and the negative symptom score of the PANSS (upward movements: −0.67; downward movements: −0.67). The correlation coefficients were not significantly correlated with the BPRS or the positive symptom score of the PANSS. The peak accelerations were not significantly correlated with any of the clinical scores. There was no significant correlation between the behavioral measures of our small sample of drug naive subjects with schizophrenia and any of the clinical scores.
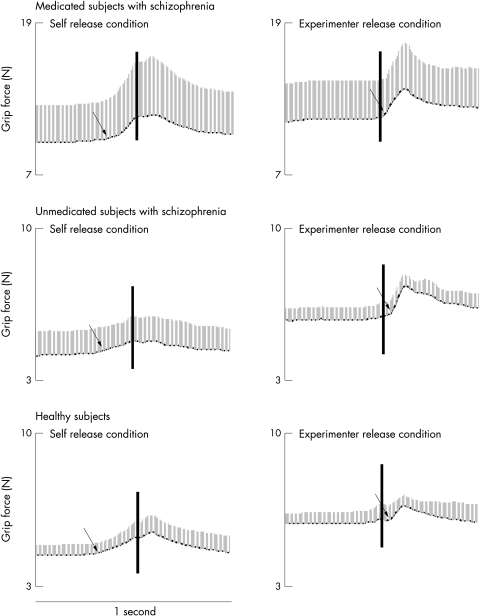
Weight catching trials
Figure 5 illustrates average traces of grip force obtained from weight catching trials performed by each group. The vertical lines within the grip force panels indicate the time of weight impact. It is evident that, irrespective of antipsychotic drugs, subjects with schizophrenia increased grip force before the time of impact in the self release condition, suggesting predictive force control. In the experimenter release condition, grip force lagged some 100 ms behind the perturbation at impact, suggesting long loop reactive force responses initiated by sensory feedback from the grasping fingers and transferred via the cerebral cortex.14
Figure 5 Average grip force traces (1 SD) for the groups of medicated subjects with schizophrenic, unmedicated subjects with schizophrenia, and healthy controls obtained from weight catching trials in the self release and experimenter release conditions. The vertical lines show the time of weight impact. The arrows show the time of grip force increase.
The peak grip forces of medicated subjects with schizophrenia were greater than those of healthy subjects (F1,24 = 31.2; p<0.001). The peak grip forces were greater in the experimenter release than in the self release condition (F1,24 = 33.6; p<0.001). The interaction “group” × “condition” was significant (F1,24 = 5.5; p<0.04), suggesting that peak grip forces were most pronounced for weight catching trials of medicated subjects in the experimenter release condition (p<0.01 for each comparison). The peak grip forces generated by healthy controls and unmedicated subjects with schizophrenia were not significantly different. However, peak grip forces were significantly greater in the experimenter release than in the self release condition (F1,8 = 28.3; p<0.01). The interaction “group” × “condition” was not significant.
Only the peak grip forces of medicated subjects with schizophrenia were significantly correlated (p<0.01 for each comparison) with the UPDRS motor subscore (self release condition: r2 = 0.80; experimenter release condition: r2 = 0.72) and the negative symptom score of the PANSS (self release condition: r2 = 0.82; experimenter release condition: r2 = 0.71), regardless of the experimental condition.
To test the hypothesis that predictive and reactive control mechanisms were maintained in subjects with schizophrenia, regression analyses between grip force traces obtained from subjects with schizophrenia with/without medication and healthy were performed. There was no significant correlation between the force traces obtained from the self release and experimenter release conditions for healthy subjects (r2 = 0.38), medicated (r2 = 0.34) and unmedicated subjects with schizophrenia (r2 = 0.27). The grip force traces of medicated (self release condition: r2 = 0.98; p<0.001; experimenter release condition: r2 = 0.99; p<0.001) and unmedicated subjects with schizophrenia (self release condition: r2 = 0.97; p<0.001; experimenter release condition: r2 = 0.92; p<0.001) were significantly correlated with the force traces of healthy subjects. These data suggest that subjects with schizophrenia adjusted grip force in a predictive manner in the self release condition and in a reactive manner in the experimenter release condition, regardless of whether they were taking antipsychotic drugs or not.
Discussion
This study investigated the impact of extrapyramidal side effects related to antipsychotic treatment on the control of grasping in schizophrenia. Drug naive subjects with schizophrenia performed similar to healthy controls. In contrast, subjects with schizophrenia receiving antipsychotic medication exhibited deficits in the scaling and timing of grip force that were strongly correlated with both the severity of extrapyramidal side effects and psychiatric negative symptoms.
Subjects with schizophrenia receiving antipsychotic treatment generated slower accelerations of the arm and excessive grip forces during both the arm movement and weight catching tasks. Sensory information from cutaneous mechanoreceptors at the grasping fingers is crucial for the accurate scaling of grip force.12,15 Indeed, an overflow in grip force has been found primarily in disorders affecting the processing of sensory feedback at a peripheral or central level.15,20 Interestingly, a slowing of the arm movement and excessive grip forces are consistent findings in subjects with Parkinson's disease.12,21 The cardinal features of Parkinson's disease result from a well circumscribed dopaminergic deficit within the basal ganglia circuits.22 The grip force overflow in Parkinson's disease has been interpreted to reflect deficient integration of afferent information from the grasping fingers at the level of the basal ganglia.21,23
Basal ganglia dysfunction attributable to blockage of dopamine‐D2 receptors is a common side effect of antipsychotic treatment in schizophrenia.4,24 Parkinson‐like symptoms were evident in any of our medicated subjects with schizophrenia. Importantly, the amount of grip force overshoot was significantly correlated with the severity of extrapyramidal symptoms as assessed by the UPDRS motor subscore. It seems as if the amount of force overshoot is a sensitive measure of extrapyramidal side effects related to antipsychotic therapy. Nevertheless, further studies investigating the effects of antipsychotic therapy over time are needed to prove this suggestion. Drug naive subjects with schizophrenia performed similar to healthy controls. One possible interpretation is that the observed performance deficits in medicated subjects are associated with antipsychotic treatment, but not a direct result of the underlying disorder. However, given the shorter disease duration within the group of drug naive subjects an alternative explanation is that the motor disability develops over the course of the disease.
Interestingly, the amount of grip overflow was also correlated with the negative symptoms of schizophrenia as assessed by the PANSS. Such correlation may, at least in part, result from a phenomenological overlap between extrapyramidal symptoms, such as slowness of movement and speech, reduced facial expression and muscular rigidity, and some of the rated negative symptoms, such as blunted affect, poor rapport, and lack of spontaneity. Indeed, there was a significant correlation between the UPDRS motor score and the negative symptom score of the PANSS. The question if the rating of negative symptoms was tainted by extrapyramidal side effects or is directly correlated with motor disability should be elucidated in future work.
We were able to confirm previous findings that subjects with schizophrenia in principle retain the ability to exhibit both predictive and reactive control mechanisms when manipulating objects.5,19 Nevertheless, the normally very precise13 temporospatial coupling between grip and load force profiles during transport movements was significantly impaired in medicated subjects. Again, the degree of disability was highly correlated with the severity of both negative symptoms of schizophrenia and extrapyramidal side effects. On the other hand, our data contrast with earlier studies on hand motor performance in schizophrenia suggesting a primary motor deficit in schizophrenia.8,9 One possible explanation for this obvious difference may be the small case number within our group of drug naive subjects.
First generation antipsychotic drugs are high affinity antagonists of dopamine‐D2 receptors that are most effective against psychotic symptoms, but have high rates of extrapyramidal side effects. Second generation antipsychotic drugs differ pharmacologically from the first generation agents in their lower affinity for dopamine D2‐receptors, which is widely accepted to cause less severe extrapyramidal side effects.24,25 All medicated subjects in our cohort received second generation drugs. Our study, however, is limited by the low case number and therefore we cannot comment on the question of how the severity of grip force overshoot and dyscoordination differs in between various second generation antipsychotic drugs depending on their neurotransmitter receptor affinity.
Our preliminary data suggest that hand motor dysfunction in schizophrenia may, at least in part, be a consequence of extrapyramidal side effects related to antipsychotic treatment. The measurement of grip forces seems to be a sensitive adjunctive method to the clinical rating of drug induced side effects in schizophrenia.
Abbreviations
PANSS - positive and negative symptom score of schizophrenia
BPRS - brief psychiatric rating scale
UPDRS - unified Parkinson's disease rating scale
Footnotes
Funding: none.
Competing interests: none declared
References
- 1.Bleuler E.Dementia praecox or the group of schizophrenia (1908). (Translated by Zinkin J). New York: International Universities Press, 1950
- 2.Kraepelin E.Dementia praecox and paraphrenia (1919). (Translated by Barclay RM; edited by Robertson GM). New York: Robert E Krieger, 1971
- 3.Vaughan S, Oquendo M, Horwath E. A patient's psychotic interpretation of a drug side effect. Am J Psychiatry 1991148393–394. [DOI] [PubMed] [Google Scholar]
- 4.Farde L, Nordström A L, Wiesel F A.et al Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine, relation to extrapyramidal side effects. Arch Gen Psychiatry 199249538–544. [DOI] [PubMed] [Google Scholar]
- 5.Delevoye‐Turrell Y, Giersch A, Danion J M. Abnormal sequencing of motor actions in patients with schizophrenia: evidence from grip force adjustments during object manipulation. Am J Psychiatry 2003160134–141. [DOI] [PubMed] [Google Scholar]
- 6.Feinberg I, Guazzelli M. Schizophrenia—a disorder of the corollary discharge systems that integrate the motor systems of thought with the sensory systems of consciousness. Br J Psychiatry 1999174196–204. [DOI] [PubMed] [Google Scholar]
- 7.Frith C, Rees G, Friston K. Psychosis and the experience of self: brain systems underlying self‐monitoring. Ann N Y Acad Sci 199815170–178. [DOI] [PubMed] [Google Scholar]
- 8.Tigges P, Mergl R, Frodl T.et al Digitized analysis of abnormal hand‐motor performance in schizophrenic patients. Schizophr Res 200029133–143. [DOI] [PubMed] [Google Scholar]
- 9.Putzhammer A, Perfahl M, Pfeiff L.et al Performance of diadochokinetic movements in schizophrenic patients. Schizophr Res 200579271–280. [DOI] [PubMed] [Google Scholar]
- 10.Boks M P M, Russo S, Knegtering R.et al The specifity of neurological signs in schizophrenia: a review. Schizophr Res 200043109–116. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan J R, Johansson R S. Hand movements. In: Ramshandran VS, ed. Encyclopedia of the human brain. Vol 2. San Diego: Academic Press, 2002399–414.
- 12.Nowak D A, Hermsdörfer J. Grip force behavior during object manipulation in neurological disorders. Toward an objective evaluation of manual performance deficits. Mov Disord 20052011–25. [DOI] [PubMed] [Google Scholar]
- 13.Flanagan J R, Wing A M. Modulation of grip force with load force during point‐to‐point arm movements. Exp Brain Res 199395131–143. [DOI] [PubMed] [Google Scholar]
- 14.Johansson R S, Westling G. Programmed and triggered actions to rapid load changes during precision grip. Exp Brain Res 19887172–86. [DOI] [PubMed] [Google Scholar]
- 15.Witney A G, Wing A, Thonnard J L.et al The cutaneous contribution to adaptive precision grip. Trends Neurosci 200427637–643. [DOI] [PubMed] [Google Scholar]
- 16.Overall J E, Gorham D R. The brief psychiatric rating scale. Psychol Rep 196210799–812. [Google Scholar]
- 17.Kay S R, Fiszbein A, Opler L A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 198713261–276. [DOI] [PubMed] [Google Scholar]
- 18.Fahn S, Elton R L. The unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne CB, et al, eds. Recent developments in Parkinson's disease. Florham Park, NJ: MacMillan Healthcare Information, 1987153–163.
- 19.Carnahan H, Elliot D, Velamoor V R. Influence of object size on prehension in leukotomized and unleukotomized individuals with schizophrenia. J Clin Exp Neuropsychol 19968136–147. [DOI] [PubMed] [Google Scholar]
- 20.Hermsdörfer J, Hagl E, Nowak D A. Deficits of anticipatory grip force control after damage to peripheral and central sensorimotor systems. Hum Mov Sci 200423643–662. [DOI] [PubMed] [Google Scholar]
- 21.Fellows S J, Schwarz M, Noth J. Precision grip in Parkinson's disease. Brain 19981211771–1784. [DOI] [PubMed] [Google Scholar]
- 22.Wichmann T, DeLong M R. Pathophysiology of parkinsonian motor abnormalities. Adv Neurol 19936053–61. [PubMed] [Google Scholar]
- 23.Gordon A M, Ingvarsson P E, Forssberg H. Anticipatory control of manipulative forces in Parkinson's disease. Exp Neurol 1997145477–488. [DOI] [PubMed] [Google Scholar]
- 24.Farde L, Wiesel F A, Nordstrom A L.et al D1‐ and D2‐dopamine receptor occupancy during treatment with conventional and atypical neuroleptics. Psychopharmacology 198999S28–S31. [DOI] [PubMed] [Google Scholar]
- 25.Miyamoto S, Duncan G E, Marx G E.et al Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 20051079–104. [DOI] [PubMed] [Google Scholar]