Abstract
Background
The high coincidence of organic vestibular and somatoform vertigo syndromes has appeared to support pathogenic models showing a strong linkage between them. It was hypothesised that a persisting vestibular dysfunction causes the development of anxiety disorders.
Objective
To determine the relation between vestibular deficits and somatoform vertigo disorders in an interdisciplinary prospective study.
Methods
Participants were divided into eight diagnostic groups: healthy volunteers (n = 26) and patients with benign paroxysmal positioning vertigo (BPPV, n = 11), vestibular neuritis (n = 11), Menière's disease (n = 7), vestibular migraine (n = 15), anxiety (n = 23), depression (n = 12), or somatoform disorders (n = 22). Neuro‐otological diagnostic procedures included electro‐oculography with rotatory and caloric testing, orthoptic examination with measurements of subjective visual vertical (SVV) and ocular torsion, and a neurological examination. Psychosomatic diagnostic procedures comprised interviews and psychometric instruments.
Results
Patients with BPPV (35.3%) and with vestibular neuritis (52.2%) had pathological test values on caloric irrigation (p<0.001). Otolith dysfunction with pathological tilts of SVV and ocular torsion was found only in patients with vestibular neuritis (p<0.001). Patients with Menière's disease, vestibular migraine, and psychiatric disorders showed normal parameters for vestibular testing but pathological values for psychometric measures. There was no correlation between pathological neurological and pathological psychometric parameters.
Conclusions
High anxiety scores are not a result of vestibular deficits or dysfunction. Patients with Menière's disease and vestibular migraine but not vestibular deficits showed the highest psychiatric comorbidity. Thus the course of vertigo syndromes and the possibility of a pre‐existing psychopathological personality should be considered pathogenic factors in any linkage between organic and psychometric vertigo syndromes.
Keywords: dizziness, vertigo, migraine, anxiety, depression
Nearly half the patients presenting at specialised units for dizziness or otoneurological disorders have psychiatric symptoms.1,2,3,4,5 These disorders are highly significant in patients with the key symptoms of dizziness, complex forms of tinnitus, or sudden and relapsing hearing loss. Sixty per cent of patients with “persisting vestibular symptoms” had persisting anxiety symptoms. In contrast to these reports, Stein and co‐workers found no association between the diagnosis of vestibular disorders and symptoms of anxiety in a dizziness clinic population; however, not all of their patients underwent vestibular testing.5
“Vestibular dysfunction” has been thought to trigger or cause anxiety syndromes owing to dysfunctional neuronal circuitry in areas such as the hippocampus, amygdala, and infralimbic cortex. The vestibular system and the neural circuitry involved in anxiety have the following links in common: the monoaminergic inputs to the vestibular system mediate effects of anxiety on vestibular function; the parabrachial nucleus network mediates emotional responses related to disordered vestibular function; and the widely distributed noradrenergic outflow from the locus coeruleus mediates the responsiveness of these symptoms to novel stimuli.6 Vestibular and visceral information, as well as somatic nociceptive inputs, converge in the parabrachial nucleus, which has reciprocal connections to the central nucleus of the amygdala and the infralimbic cortex and is under the control of higher cortical cognitive regions. Furthermore, close connections between the vestibular system and autonomic functions and respiration were described in animal studies and more recently also in humans.7,8,9 A linkage of the vestibular system and anxiety disorders was only recently hypothesised in rats.10 The findings in humans show that a bilateral vestibular lesion has a significant effect on the hippocampus.11 Indeed, rats also showed behavioural abnormalities six months after bilateral vestibular damage.10 As such damage may be related to disturbances in the emotional and cognitive processing centre of the hippocampus, it was hypothesised that anxiety and other emotional changes are direct consequences of a vestibular lesion in the hippocampus.
On the other hand, a strong comorbidity between psychiatric disorders and vestibular dysfunction, especially for anxiety disorders, has been reported.4,5,12,13 Vestibular abnormalities have often been reported in patients with psychiatric disorders. The results of vestibular tests used to measure these abnormalities, such as caloric irrigation and dynamic posturography, have often been interpreted as indicating significant impairment in patients with anxiety symptoms—for example, a surface dependence during balance control in patients with panic disorders with agoraphobia.14
It is an open question, however, whether such abnormalities are of pathological relevance, represent an earlier transient dysfunction that can be compensated, or signal a change in normal postural control strategies. The latter explanation has been supported by a series of studies analysing the parameters of body sway in patients with somatoform phobic postural vertigo. These studies showed an increased body sway during easy balance conditions. The patients constantly monitored their balance and produced a “stiffening up” strategy by coactivating antigravity muscles, a strategy similar to that of healthy subjects when performing demanding balance tasks.15,16,17,18 This raises the question of whether vestibular organic disorders with pathological clinical relevance or vestibular dysfunctions without clinical relevance really underlie psychiatric disorders.
We designed a study to determine, first, whether vestibular disorders can induce psychiatric disorders; second, whether there is a correlation between abnormal vestibular and psychometric test values suggesting that vestibular pathologies are able to induce psychological pathologies3; or third, whether it is coincidence that vestibular disorders are often associated with psychiatric disorders.
Methods
In a prospective interdisciplinary study, 127 patients with different vertigo syndromes and healthy subjects were included consecutively. Demographic data are shown in table 1. After giving their informed written consent, the patients were examined in the departments of neurology and psychosomatic medicine and psychotherapy. The study was approved by the local ethics committee.
Table 1 Demographic data.
| Group | Age (years) | Sex | |||||
|---|---|---|---|---|---|---|---|
| n | % | Mean | SD | Range | Female | Male | |
| Healthy controls | 26 | 20.3 | 33.00 | 13.35 | 22 to 60 | 13 | 13 |
| BPPV | 11 | 8.6 | 56.00 | 11.39 | 36 to 71 | 6 | 5 |
| Vestibular neuritis | 11 | 8.6 | 50.72 | 12.12 | 28 to 68 | 4 | 7 |
| Menière's disease | 7 | 5.5 | 61.00 | 11.49 | 42 to 73 | 3 | 4 |
| Vestibular migraine | 15 | 11.7 | 46.13 | 8.12 | 35 to 61 | 9 | 6 |
| Anxiety | 23 | 18.0 | 44.96 | 13.42 | 23 to 69 | 18 | 5 |
| Depression | 12 | 9.4 | 43.50 | 14.56 | 22 to 67 | 7 | 5 |
| Somatoform | 22 | 17.2 | 44.14 | 12.78 | 23 to 68 | 7 | 15 |
| Total | 127 | 100 | 44.67 | 14.33 | 22 to 73 | 67 | 60 |
BPPV, benign paroxysmal positioning vertigo.
Subjects were divided into three groups:
patients with organic disorders caused by benign paroxysmal positioning vertigo (BPPV), vestibular neuritis, Menière's disease, or vestibular migraine;
patients with somatoform vertigo caused by phobic and anxiety disorders, depressive disorders, or somatoform disorders;
a group of healthy controls.
Healthy subjects were recruited from our hospital staff and by public announcements. Patients were recruited from our outpatient clinic and from the wards. Those with Menière's disease, BPPV, or vestibular migraine were enrolled in the study if the episode leading to the diagnosis had occurred within the past six months. Patients with vestibular neuritis were enrolled within the first seven days after disease onset.
BPPV was diagnosed by the typical complaints and a positive Dix‐Hallpike head positioning test. Patients with complaints of positioning vertigo but with a negative Dix‐Hallpike head positioning test were not included. Vestibular neuritis was diagnosed by the typical history (rotating vertigo, nausea, oscillopsia, gait imbalance) and neurological findings (spontaneous nystagmus, ipsilateral body tilts, pathological head impulse test; number of signs of a central disorder); the clinical diagnosis was confirmed by neuro‐otological findings (ipsilateral ocular torsion, tilts of the subjective visual vertical (SVV), ipsilateral caloric hyporesponsiveness). Menière's disease was diagnosed according to the diagnostic criteria of the American Academy of Ophthalmology and Otolaryngology.19 Vestibular migraine was diagnosed in patients with episodic vestibular symptoms, if they had a positive history or positive family history of migraine, if migrainous symptoms had occurred during at least two attacks of vertigo, if migraine precipitants had occurred before vertigo, or if there was a positive response to migraine medications.
Exclusion criteria were acute or chronic central vestibular and central ocular motor disorders, regular intake of drugs affecting the CNS, ongoing psychotherapy, an acute psychotic disorder, an acute disease of the CNS, or an acute malignancy.
Neurological and neuro‐otological examinations
All participants underwent detailed diagnostic procedures consisting of neurological and neuro‐otological examinations including positioning manoeuvres, stepping test, head impulse test, examination with Frenzel's glasses, and head shaking test. Fundus photographs to detect tonic vestibular disorders, adjustments of SVV to measure otolith dysfunction, and binocular electro‐oculography (EOG), including rotatory and caloric testing to measure semicircular canal dysfunction, were carried out. The following variables were chosen for further statistical analysis: side differences in caloric testing, binocular tilts of SVV, and measurements of binocular torsion. The other variables were within normal range for all subgroups. All patients completed the full battery of neurological and neuro‐otological tests to confirm the clinical diagnosis made by the neurologist/psychiatrist.
Electro‐oculography
Complete testing included spontaneous nystagmus, fixation straight ahead, and gaze evoked nystagmus up to ±40° of lateral gaze.
Voluntary saccades
Mean peak velocities were evaluated from six saccades. Hypometric (undershooting) saccades were considered abnormal, as constant dysmetria exceeding at least 20% of the amplitude; the lower limit for saccadic mean velocity was 300°/s.
Optokinetic nystagmus
An alternating black and white stripe pattern was moved across the screen. The gain was evaluated from 10 responses and was considered within normal range between a gain of 0.7 and 1.0.
Smooth pursuit
The stimulus was a sinusoidally moving laser spot. Impairment was manifested as an interruption by saccades.
Rotatory testing
Subjects were seated on a rotatory chair with eyes closed. The chair was accelerated, kept constant for 90 seconds, and then stopped. Per‐rotatory and post‐rotatory nystagmus were determined by slow phase velocity, duration, and gain.
Caloric irrigation
Vestibular paresis/dysfunction on caloric irrigation according to Honrubia was defined as an asymmetry of more than 25% between right sided and left sided responses.20 Asymmetry was calculated by the formula of Jongkees from the slow phase velocity of response nystagmus: {[(R30°+R44°) − (L30°+L44°)]/[R30°+R44°+L30°+L44°]} × 100, expressed as a percentage. Spontaneous nystagmus that could not be suppressed by fixation was classified as pathological (above 5°/s).
Subjective visual vertical
SVV was measured to determine acute otolith dysfunction.21 Subjects sat in an upright position looking into a hemispheric dome, rotating about the line of sight. The surface of the dome was covered with a random pattern of coloured dots containing no cues to gravitational orientation. Subjects had to adjust the target disk to the vertical. Static SVV was determined by means of seven adjustments from a random offset position with the hemispherical dome stationary.21 Under these conditions, the normal range (±2 SD) of the SVV is ±2.5°.22,23 The static SVV was determined both binocularly and monocularly.21,24,25,26
Ocular torsion
Fundus photographs for measurements of tonic ocular torsion as a sign of otolith dysfunction were made using a scanning laser ophthalmoscope. Ocular torsion, in degrees, was defined as the mean of four to six fundus photographs. The position of the eye in the roll plane was determined as the angle between a straight line through papilla and macula and the horizontal line. With this method, the normal eye position for both eyes is an excyclotropia of around 5° (for details see Dieterich and Brandt21 and Curthoys et al27). A difference of more than 8° between the degree of torsion of both eyes was defined as pathological.
Psychometric examination
Besides clinical psychosomatic diagnostic procedures (one to three clinical interviews), the following psychometric instruments were used:
Vertigo symptom scale
The VSS assesses symptoms of balance system dysfunction (for example, dizziness, vertigo, postural instability, and falling) and symptoms of somatic anxiety and autonomic arousal (diverse pains and somatic sensations, sweating, pounding heart, breathing difficulties, and fainting).28
Vertigo handicap questionnaire
The VHQ comprises 25 statements about the handicapping consequences of vertigo, ranging from difficulty to perform various physical activities to interference with social relations and leisure pursuits.
Hospital anxiety and depression scale
The HADS is a self rating questionnaire used to measure anxiety and depression. The HADS, given in the German version, is a reliable and validated psychometric instrument which has also been used in a controlled trial in a vertigo population.29 Thus values over 10 for anxiety and over 8 for depression correspond to a significant distress increase.
Screening for somatoform disorders
The SOMS is a screening instrument for somatoform disorders and somatisation tendencies, which records symptoms of somatisation and criteria for the diagnosis of a somatoform disorder according to DSM‐IV and ICD‐10.
SCID
The structural clinical interview for DSM‐IV axis I disorders (SCID‐I) in its German version has been used since 1997 for patients with psychiatric disorders.30 Based on the DSM‐IV criteria, it includes the main psychiatric syndromes and disorders including depression, anxiety disorders, psychoticism, and somatoform disorders.
Statistical analysis
Analysis of differences started with a single factor multivariate analysis of variance (MANCOVA) that also generated univariate analysis of variance (ANOVA) followed by pairwise post hoc tests (LSD). Age was introduced as a covariate. Dependent variables were six psychometric (VHQ, VSS‐Severity, VSS‐Anxiety, HADS‐Anxiety and Depression, SOMS) and three neurological variables (side difference of caloric testing, tilts of SVV, binocular torsion). Pearson correlations between neurological and psychometric variables were tested against a correlation of 0. All computations were carried out using SPSS for Windows version 12. Alpha error was set to 5%.
Results
In all, 127 subjects with complete datasets were included in the analysis (table 1).
Three of the BPPV patients had a history of vestibular neuritis at least six months before the study and were included in the BPPV subgroup. All patients diagnosed as having vestibular neuritis showed spontaneous nystagmus during the first clinical examination, pathological head‐impulse test, and ipsilateral body tilt. Only eight of these patients had significant unilateral caloric hypo/unresponsiveness. The other three had hyporesponsiveness of 17%–21%, which was close but below the criteria of at least 25% side difference. As they had the typical history, ipsilateral SVV tilts, and ocular torsion, they were included in the subgroup of vestibular neuritis. None of the patients had signs or symptoms of more than one acute vestibular disorder. Of the vestibular migraine patients, four of 15 (27%) presented with minor and isolated central ocular motor signs (upward gaze evoked nystagmus (n = 1), dissociated pendular nystagmus (n = 1), and saccadic smooth pursuit (n = 2)). Four other patients had significant strabismus, which prevented an unambiguous classification. As all other examinations were within the normal range, we did not interpret these findings as pathological.
The mean age of the healthy controls was 33, a younger age than the mean age of the patient subgroups (table 1). Age was therefore entered as a covariate in the multivariate analysis; thus the influence of age on analysis variables was subtracted before the analyses were undertaken.
Multivariate tests showed significant differences between the eight subgroups (F = 6.02, df = 140; 742, p<0.001) (table 2).
Table 2 Statistical tests for all test parameters.
| MANOVA/ANOVA (F tests) | ||||
|---|---|---|---|---|
| F | df1 | df2 | p Value | |
| Multivariate | 6.015 | 140 | 742 | 0.000 |
| EOG‐SPN | 0.322 | 7 | 119 | 0.943 |
| Calorics, side difference | 5.592 | 7 | 119 | 0.000 |
| Tilts of SVV | 12.044 | 7 | 119 | 0.000 |
| OT, difference | 5.411 | 7 | 119 | 0.000 |
| VHQ | 19.401 | 7 | 119 | 0.000 |
| VSS | ||||
| Anxiety | 11.593 | 7 | 119 | 0.000 |
| Severity | 12.950 | 7 | 119 | 0.000 |
| HADS | ||||
| Anxiety | 12.881 | 7 | 119 | 0.000 |
| Depression | 11.753 | 7 | 119 | 0.000 |
| SOMS | 11.063 | 7 | 119 | 0.000 |
EOG‐SPN, spontaneous nystagmus on electro‐oculography; HADS, hospital anxiety and depression scale; OT, ocular torsion; SOMS, screening for somatoform disorders; SVV, subjective visual vertical; VHQ, vertigo handicap questionnaire; VSS, vertigo symptom scale.
Post hoc pairwise test results are given in table 3.
Table 3 Statistical tests with subgroup comparison for all test parameters.
| Group differences | Post hoc LSD tests for group differences (p values) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Calorics difference | OT difference | SVV | VSS A | VSS S | VHQ | HADS A | HADS D | SOMS | |
| HC–BPPV | 0.001 | 0.723 | 0.043 | 0.134 | 0.000 | 0.000 | 0.179 | 0.224 | 0.065 |
| HC–VN | 0.000 | 0.000 | 0.000 | 0.090 | 0.014 | 0.020 | 0.021 | 0.013 | 0.018 |
| HC–MD | 0.331 | 0.804 | 0.526 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| HC–VM | 0.752 | 0.860 | 0.119 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 |
| HC–A | 0.841 | 0.300 | 0.733 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| HC–D | 0.129 | 0.880 | 0.788 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| HC–S | 0.086 | 0.582 | 0.079 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| BPPV–VN | 0.126 | 0.000 | 0.000 | 0.867 | 0.292 | 0.190 | 0.410 | 0.276 | 0.647 |
| BPPV–MD | 0.104 | 0.630 | 0.337 | 0.026 | 0.419 | 0.120 | 0.002 | 0.018 | 0.024 |
| BPPV–VM | 0.006 | 0.860 | 0.566 | 0.001 | 0.004 | 0.204 | 0.013 | 0.079 | 0.000 |
| BPPV–A | 0.002 | 0.643 | 0.084 | 0.000 | 0.017 | 0.000 | 0.000 | 0.000 | 0.000 |
| BPPV–D | 0.108 | 0.857 | 0.126 | 0.003 | 0.782 | 0.018 | 0.000 | 0.000 | 0.052 |
| BPPV–S | 0.058 | 0.931 | 0.546 | 0.019 | 0.405 | 0.029 | 0.002 | 0.009 | 0.050 |
| VN–MD | 0.003 | 0.000 | 0.000 | 0.038 | 0.084 | 0.007 | 0.018 | 0.156 | 0.065 |
| VN–VM | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 | 0.008 | 0.105 | 0.552 | 0.002 |
| VN–A | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 |
| VN–D | 0.002 | 0.000 | 0.000 | 0.005 | 0.177 | 0.000 | 0.007 | 0.001 | 0.137 |
| VN–S | 0.000 | 0.000 | 0.000 | 0.031 | 0.042 | 0.000 | 0.027 | 0.164 | 0.150 |
| MD–VM | 0.496 | 0.722 | 0.064 | 0.550 | 0.099 | 0.584 | 0.268 | 0.324 | 0.450 |
| MD–A | 0.408 | 0.351 | 0.690 | 0.230 | 0.253 | 0.064 | 0.454 | 0.230 | 0.422 |
| MD–D | 0.805 | 0.739 | 0.711 | 0.684 | 0.563 | 0.609 | 0.987 | 0.115 | 0.561 |
| MD–S | 0.843 | 0.541 | 0.577 | 0.633 | 0.848 | 0.895 | 0.446 | 0.691 | 0.399 |
| VM–A | 0.892 | 0.470 | 0.218 | 0.459 | 0.425 | 0.002 | 0.013 | 0.004 | 0.997 |
| VM–D | 0.269 | 0.990 | 0.286 | 0.836 | 0.008 | 0.204 | 0.197 | 0.002 | 0.110 |
| VM–S | 0.236 | 0.760 | 0.989 | 0.153 | 0.013 | 0.359 | 0.596 | 0.404 | 0.035 |
| A–D | 0.184 | 0.492 | 0.991 | 0.360 | 0.032 | 0.116 | 0.353 | 0.513 | 0.082 |
| A–S | 0.139 | 0.644 | 0.166 | 0.016 | 0.054 | 0.013 | 0.030 | 0.022 | 0.018 |
| D–S | 0.930 | 0.765 | 0.245 | 0.266 | 0.593 | 0.604 | 0.368 | 0.011 | 0.802 |
A, anxiety; BPPV, benign paroxysmal positioning vertigo; D, depression; HADS, hospital anxiety and depression scale; HC, healthy control; MD, Menière's disease; OT, ocular torsion; S, somatoform disorders; SOMS, screening for somatoform disorders; SVV, subjective visual vertical; VHQ, vertigo handicap questionnaire; VM, vestibular migraine; VN, vestibular neuronitis; VSS, vertigo symptom scale.
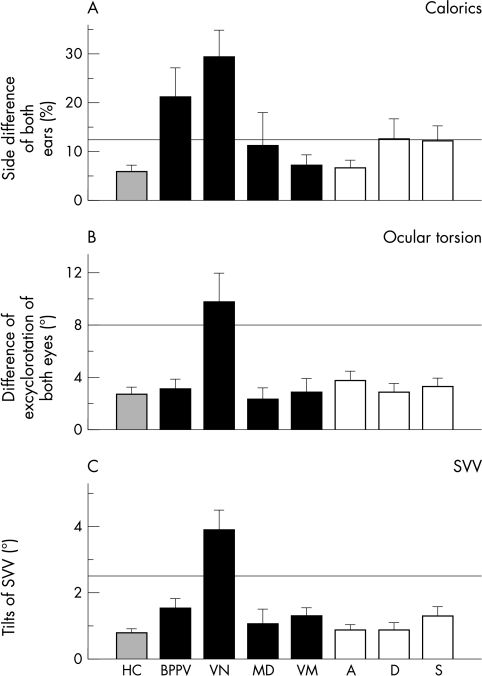
Pathological results were detected with EOG and caloric testing only for the patients with vestibular neuritis and BPPV (fig 1A). Differences between the patients with BPPV and vestibular neuritis compared with the other groups were significant (F = 5.592, df = 7; 119, p<0.001). Of all patients with BPPV three had a history of previous vestibular neuritis, which explained the pathological findings for the side difference detected by caloric testing. All three patients showed unilateral caloric unresponsiveness. Values within the normal range were found for all other subgroups.
Figure 1 Results of neuro‐otological examinations. (A) Side difference of both ears (%) during caloric irrigation. (B) Difference of excyclorotation between the eyes (°) assessed by fundus photographs (normal ⩽8°). (C) Tilts of the subjective visual vertical, SVV (°) (normal ⩽2.5°). Normal ranges are given by vertical lines above the bars. SVV, subjective visual vertical.
Measurements of ocular torsion and tilts of SVV also showed pathological results for the patients with vestibular neuritis (fig 1, panels B and C); values were within the normal range for all other subgroups. Differences were also significant (F = 5.411, df = 7;119, p<0.001; F = 12.044, df = 7;119, p<0.001).
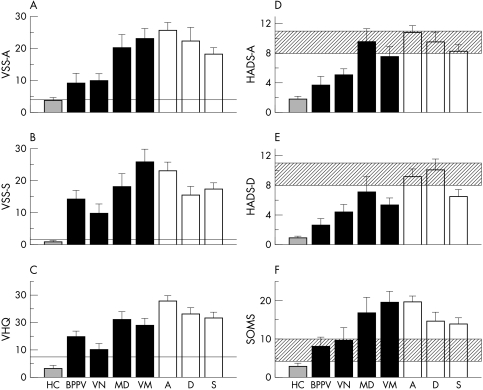
The greatest amount of anxiety, measured by the VSS, was found in the subgroups of patients with anxiety and depressive disorders. Interestingly, high anxiety values were also seen in patients with vestibular migraine and Menière's disease (fig 2A). Patients with somatoform and anxiety disorders had the greatest severity of vertigo symptoms according to the VSS. This was also true for patients with vestibular migraine and Menière's disease (fig 2B). According to the vertigo handicap questionnaire, patients with vestibular migraine and Menière's disease and all somatoform vertigo subgroups had the highest vertigo induced handicap (fig 2C).
Figure 2 Results of psychosomatic examinations. (A) Symptom‐anxiety according to the vertigo symptom scale (VSS‐A). (B) Symptom‐severity according to the vertigo symptom scale (VSS‐B). (C) Handicap caused by vertigo according to vertigo handicap scale (VHQ). Values of 0 represent “not at all”, values of 100 “very high”. (D)–(F): Results of psychosomatic examinations. (D) Anxiety according to HADS‐A (low 0, high 21). (E) Depression according to HADS‐D (low 0, high 21). (F) Somatisation according to the screening test for somatoform disorders (SOMS) score (low 0, high 52). HADS, hospital anxiety and depression scale.
The HADS‐A showed the highest values for anxiety in patients with somatoform and depressive disorders and in patients with Menière's disease (fig 2D). Patients with somatoform and depressive disorders and also patients with Menière's disease (fig 2E) had the highest values for depression according to the HADS‐D. Highest values for somatisation according to the SOMS were found in patients with Menière's disease, vestibular migraine, and anxiety disorders (fig 2F). There was no correlation between the parameters of the vestibular and the psychometric tests (table 4).
Table 4 Analysis of correlations between the parameters of vestibular testing and the parameters of psychometric testing.
| Test | EOG‐SPN | Calorics, side difference | Tilts of SVV | OT, difference | |
|---|---|---|---|---|---|
| VHQ | P* | −0.097 | 0.024 | −0.083 | −0.063 |
| S† | 0.278 | 0.786 | 0.351 | 0.481 | |
| n‡ | 127 | 127 | 127 | 127 | |
| VSS | |||||
| Anxiety | P | 0.011 | −0.156 | −0.047 | 0.044 |
| S | 0.900 | 0.079 | 0.599 | 0.625 | |
| n | 127 | 127 | 127 | 127 | |
| Severity | P | −0.129 | 0.037 | −0.014 | 0.070 |
| S | 0.146 | 0.680 | 0.878 | 0.434 | |
| n | 127 | 127 | 127 | 127 | |
| HADS | |||||
| Anxiety | P | 0.018 | −0.071 | −0.018 | −0.012 |
| S | 0.841 | 0.423 | 0.838 | 0.894 | |
| n | 127 | 127 | 127 | 127 | |
| Depression | P | −0.011 | 0.055 | 0.051 | 0.036 |
| S | 0.903 | 0.536 | 0.565 | 0.683 | |
| n | 127 | 127 | 127 | 127 | |
| SOMS | P | 0.019 | −0.096 | −0.083 | 0.040 |
| S | 0.828 | 0.281 | 0.350 | 0.654 | |
| n | 127 | 127 | 127 | 127 | |
*Pearson's correlation.
†Significance.
‡Number of cases.
EOG‐SPN, spontaneous nystagmus on electro‐oculography; HADS, hospital anxiety and depression scale; OT, ocular torsion; SOMS, screening for somatoform disorders score; SVV, subjective visual vertical; VHQ, vertigo handicap questionnaire; VSS, vertigo symptom scale.
Discussion
Analysis of neuro‐otological variables showed that only patients with vestibular neuritis and BPPV had pathological values on vestibular testing. Patients with vestibular neuritis had signs of an acute vestibular lesion with otolith deficits (tilt of SVV and ocular torsion) and semicircular canal deficits (unilateral caloric hypo/unresponsiveness). Patients with BPPV showed only an asymmetry of caloric testing—that is, unresponsiveness following vestibular neuritis (in three patients) but no signs of acute vestibular dysfunction. Such a pattern in BPPV indicates an earlier vestibular deficit, which was centrally compensated and is therefore not of clinical relevance for the current vertigo syndrome. Vestibular testing of all other subgroups yielded values within the normal range (fig 1).
The distribution of age and sex was similar to that of other studies and reflects our clinical experience.31,32,33,34 Thus the patient selection in our study represents the normal distribution of epidemiological data for these disease entities.
Psychometric testing revealed that not only patients with somatoform disorders but also those with organic vestibular vertigo syndromes had pathological values. In particular the subgroups of patients with Menière's disease and vestibular migraine were found to have high values for anxiety and depression. These interesting results will be discussed elsewhere in more detail (Eckhardt‐Henn A, in preparation). A coexistence of pathological findings in psychometric scores and vestibular diseases was described earlier.1,2,3,13,14,28 Interestingly, those of our patients with vestibular disorders who had persistent vestibular deficits—for example, pathological side differences in caloric testing—did not show the highest values in psychometric scores, but instead patients with overall normal values in neurological and neuro‐otological testing (vestibular migraine and Menière's disease). Thus, in contrast to earlier studies, we found no correlation between an acute or chronic vestibular dysfunction and pathology on psychometric testing. Our results do not support the hypothesis that latent vestibular dysfunction or imbalance triggers anxiety disorders, above all agoraphobia.
Answering the question of whether this coexistence is only coincidental, our results suggested that special vestibular syndromes—for example, vestibular migraine and Menière's disease—may function as a trigger for a secondary somatoform disorder, but not vestibular disorders in general and especially not a subtle vestibular tone imbalance. To answer this question in more detail, further data on these patients need to be evaluated in a longitudinal study. However, our data represent the first evaluation of a longitudinal study with four controls over a time interval of one year.
Therefore, the question to be addressed is whether or not the development of psychosomatic pathology depends on the patient's ability to control relapses of a disorder. Patients with BPPV often learn to control their vertigo attacks by avoiding rapid head movements. This could explain the “normal” psychometric findings in these patients, and moreover, the pathological findings in patients with vestibular migraine and Menière's disease, as those disorders typically cannot be controlled by the patients. Alternatively, the different pathophysiological mechanisms of BPPV, and of Menière's disease or vestibular migraine, may explain these different findings. During BPPV attacks, symptoms related to a mechanical problem occur, and their treatment is purely mechanical (through positioning manoeuvres). In contrast, changes in neurotransmitters are involved in Menière's disease and vestibular migraine, and symptom relief is always obtained by manipulation of these neurotransmitters. As similar neurotransmitters are also involved in the processing of anxiety and depression, a close connection between these systems is likely.6,7,8
Patients with somatoform vertigo syndromes in our study showed more handicap (VHQ) and higher symptom severity and anxiety (VSS) because of dizziness than patients with BPPV and vestibular neuritis; and patients with BPPV and vestibular neuritis showed more distress than the healthy controls. No correlation was found between psychometric testing and an organic deficit in vestibular testing (table 4).
In conclusion, our results do not provide any evidence that higher anxiety scores are a result of vestibular deficits or vestibular dysfunction, as discussed in earlier studies.2,3,13,35 Some investigators believed that “non‐syndromal vestibular disorders” may cause a particular vulnerability for specific phobic disorders and in doing so maintain the associated avoidance behaviour.34,36 Other research groups argued, in contrast, that these were non‐specific findings in part based on individual outcomes and interpretations.16,17,37,38,39,40 Our findings agree with the latter arguments. For example, it could be shown that posturographic abnormalities in patients with somatoform phobic vertigo were caused by a change of strategy of stance owing to a previously present underlying anxiety.15,16,17,39
These discrepancies in the interpretation of vestibular parameters, for example EOG measurements, require explanation. One reason might be the different interpretation of single vestibular parameters outside the context of others.35 Diagnosis of a vestibular disease cannot be made on the side differences in caloric irrigation alone, a direction preponderance for caloric or rotatory testing alone, isolated spontaneous nystagmus, or abnormalities in posturography. Moreover, it should be kept in mind that approximately 20% of normal subjects have a low velocity spontaneous nystagmus measured with the eyes closed, considered as pathological only if its slow phase velocity amounts to more than 5–6°/s.41,42 Such vestibular signs should be considered relevant only if a caloric hyporesponsiveness is combined with a corresponding pathological spontaneous and gaze evoked nystagmus. The occurrence of a caloric hyporesponsiveness alone discloses an older vestibular deficit that in the meantime has been centrally compensated and is therefore not clinically relevant. We considered both aspects: acute vestibular lesions (in vestibular neuritis) and chronic vestibular signs (in BPPV). Vestibular parameters did not correlate with psychometric test parameters. We therefore cannot support the hypothesis that subtle vestibular tone imbalances have a triggering function in the development of somatoform disorders.
Our finding that patients with vertigo syndromes but without abnormalities in vestibular testing can have pathological values in psychometric test parameters agreed with findings of several studies.5,43,44 In the study by Stein and co‐workers, who examined 142 patients in part by neuro‐otological and psychometric examinations, anxiety disorders occurred with a similar frequency in patients with and without a vestibular disease.5 However, the study had a few limitations. First, only patients with positive self reported questionnaire results were selected for a diagnostic interview. Second, not all patients underwent neuro‐otological investigations, which did not include otolith testing (SVV and ocular torsion). Third, the differentiation of vestibular disorders was in part based on an incomplete neuro‐otological examination. Reactive and comorbid psychiatric disorders are known to emerge as a result of organic dizziness such as vestibular neuritis or BPPV.32 Medical experts may overlook these disorders, instead attributing symptoms to residual states following an organic lesion and thus delaying the correct diagnosis. This false conclusion is usually made on the basis of minimal neuro‐otological findings, as discussed above. If the organic disorder remains undetected, it can lead to psychological reactions such as anxiety, phobic fears, avoidance, and depressive symptoms.32,45,46
A false interpretation of irrelevant vestibular signs may elicit a iatrogenic fixation on an organic origin of the disorder and cause a secondary phobic reaction. This phobic reaction has to be differentiated from a typical reactive phobic disorder—for example, after an acute attack of vestibular neuritis. For these reasons, an interdisciplinary, differentiated diagnostic procedure and an early prophylactic intervention are recommended.
Acknowledgements
We wish to thank Anja Kühn for her help with the measurements and Judy Benson for copy editing the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (EC 220/2‐1) and Maifor.
Abbreviations
BPPV - benign paroxysmal positioning vertigo
DSM‐IV - Diagnostic and Statistical Manual of Mental Disorders, fourth edition
EOG - electro‐oculography
HADS - hospital anxiety and depression scale
OT - ocular torsion
SCID - structural clinical interview for DSM‐IV axis I disorders
SOMS - screening for somatoform disorders score
SVV - subjective visual vertical
VHQ - vertigo handicap questionnaire
VSS - vertigo symptom scale
Footnotes
Competing interests: none declared
References
- 1.McKenna L, Hallam R S, Hinchcliffe R. The prevalence of psychological disturbance in neurotology outpatients. Clin Otolaryngeol 199116452–456. [DOI] [PubMed] [Google Scholar]
- 2.Clark D B, Leslie M I, Jacob R G. Balance complaints and panic disorder: a clinical study of panic symptoms in members of a self‐help group for balance disorders. J Anxiety Disord 1992647–53. [Google Scholar]
- 3.Clark D B, Hirsch B E, Smith M G.et al Panic in otolaryngology patients presenting with dizziness or hearing loss. Am J Psychiatry 19941511223–1225. [DOI] [PubMed] [Google Scholar]
- 4.Eagger S, Luxon L M, Davies R A.et al Psychiatric morbidity in patients with peripheral vestibular disorder: a clinical and neuro‐otological study. J Neurol Neurosurg Psychiatry 199255383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein M B, Asmundson G J, Ireland D.et al Panic disorder in patients attending a clinic for vestibular disorders. Am J Psychiatry 19941511697–1700. [DOI] [PubMed] [Google Scholar]
- 6.Balaban C D, Thayer J F. Neurological bases for balance‐anxiety links. J Anxiety Disord 20011553–79. [DOI] [PubMed] [Google Scholar]
- 7.Yates B J, Miller A D. Physiological evidence that the vestibular system participates in autonomic and respiratory control. J Vestib Res 1998817–25. [PubMed] [Google Scholar]
- 8.Jauregui‐Renaud K, Gresty M A, Reynolds R.et al Respiratory responses of normal and vestibular defective human subjects to rotation in the yaw and pitch planes. Neurosci Lett 200129817–20. [DOI] [PubMed] [Google Scholar]
- 9.Radtke A, Popov K, Bronstein A M.et al Evidence for a vestibulo‐cardiac reflex in man. Lancet 2000356736–737. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Y, Horii A, Appleton I.et al Bilateral vestibular deafferentiation impairs object recognition in rat. Neuroreport 2004151913–1916. [DOI] [PubMed] [Google Scholar]
- 11.Brandt T, Schautzer F, Hamilton D.et al Spatial memory deficits and hippocampal atrophy in NF2 patients with bilateral vestibular failure. J Vestib Res 200414150 [Google Scholar]
- 12.Sklare D A, Stein M B, Pikus A M.et al Dysequilibrium and audiovestibular function in panic disorder: symptom profiles and test findings. Am J Otol 199011338–341. [PubMed] [Google Scholar]
- 13.Jacob R G, Moller M B, Turner S M.et al Otoneurological examination in panic disorder and agoraphobia with panic attacks: a pilot study. Am J Psychiatry 1985142715–720. [DOI] [PubMed] [Google Scholar]
- 14.Jacob R G, Furman J M, Joseph M.et al Surface dependence: a balance control strategy in panic disorder with agoraphobia. Psychosom Med 199759323–330. [DOI] [PubMed] [Google Scholar]
- 15.Krafczyk S, Schlamp V, Dieterich M.et al Increased body sway at 3.5–8 Hz in patients with phobic postural vertigo. Neurosci Lett 1999259149–152. [DOI] [PubMed] [Google Scholar]
- 16.Querner V, Krafczyk S, Dieterich M.et al Patients with somatoform phobic postural vertigo: the more difficult the balance task, the better the balance performance. Neurosci Lett 200028521–24. [DOI] [PubMed] [Google Scholar]
- 17.Querner V, Krafczyk S, Dieterich M.et al Phobic postural vertigo. Body sway during visually induced roll vection. Exp Brain Res 2002143269–275. [DOI] [PubMed] [Google Scholar]
- 18.De Luca C J, Mambrito B. Voluntary control of motor units in human antagonist muscles: coactivation and reciprocal activation. Neurophysiol 198758525–542. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Ophthalmology and Otolaryngology Committee on Hearing and Equilibrium guidelines for diagnosis and evaluation of therapy in Menière's disease. Otolaryngol Head Neck Surg 1995113181–185. [DOI] [PubMed] [Google Scholar]
- 20.Honrubia V. Quantitative vestibular function tests and the clinical examination. In: Herdmann SJ, ed. Vestibular rehabilitation. Philadelphia: Davis, 1994113–164.
- 21.Dieterich M, Brandt T. Ocular torsion and tilt of subjective visual vertical are sensitive brainstem signs. Ann Neurol 199333292–299. [DOI] [PubMed] [Google Scholar]
- 22.Dichgans J, Held R, Young L.et al Moving visual scenes influence the apparent direction of gravity. Science 19721781217–1219. [DOI] [PubMed] [Google Scholar]
- 23.Held R, Dichgans J, Bauer J. Characteristics of moving visual scenes influencing spatial orientation. Vis Res 197515357–365. [DOI] [PubMed] [Google Scholar]
- 24.Boehmer A, Mast F. Assessing otolith function by the subjective visual vertical. Ann NY Acad Sci 1999871221–231. [DOI] [PubMed] [Google Scholar]
- 25.Dieterich M, Brandt T. Ocular torsion and perceived vertical in oculomotor, trochlear and abducens nerve palsies. Brain 19931161095–1104. [DOI] [PubMed] [Google Scholar]
- 26.Dieterich M, Brandt T. Wallenberg's syndrome: lateropulsion, cyclorotation, and subjective visual vertical in thirty‐six patients. Ann Neurol 199231399–408. [DOI] [PubMed] [Google Scholar]
- 27.Curthoys I S, Dai M J, Halmagyi G M. Human ocular torsion before and after unilateral vestibular neurectomy. Exp Brain Res 199185218–225. [DOI] [PubMed] [Google Scholar]
- 28.Yardley L, Masson E, Verschuur C.et al Symptoms, anxiety and handicap in dizzy patients: development of the vertigo symptom scale. J Psychosom Res 199236731–741. [DOI] [PubMed] [Google Scholar]
- 29.Zigmond A S, Snaith R P. The hospital and depression scale. Acta Psychiatr Scand 199367361–367. [DOI] [PubMed] [Google Scholar]
- 30.Wittchen H U, Burke J D, Semler G.et al Recall and dating of psychiatric symptoms. Test‐retest reliability of time‐related symptom questions in a standardized psychiatric interview. Arch Gen Psychiatry 198946437–443. [DOI] [PubMed] [Google Scholar]
- 31.Strupp M, Glaser M, Karch C.et al The most common form of dizziness in middle age: phobic postural vertigo. Nervenarzt 200374911–914. [DOI] [PubMed] [Google Scholar]
- 32.Eckhardt‐Henn A, Breuer P, Thomalske C.et al Anxiety disorders and other psychiatric subgroups in patients complaining of dizziness. J Anxiety Disord 200317369–388. [DOI] [PubMed] [Google Scholar]
- 33.Baloh R W, Honrubia V. Endolymphatic hydrops (Meniere's syndrome). In: Baloh, RW, Honrubia V, editors. Clinical neurophysiology of the vestibular system. 3rd ed. New York: Oxford University Press, 2001252–263.
- 34.Baloh R W, Honrubia V. Migraine. In: Baloh, RW, Honrubia V, editors. Clinical neurophysiology of the vestibular system, 3rd edition. New York: Oxford University Press, 2001264–276.
- 35.Jacob R G, Furman J M, Durrant J D.et al Panic, agoraphobia and vestibular dysfunction. Am J Psychiatry 1996153503–512. [DOI] [PubMed] [Google Scholar]
- 36.Jacob R G, Brandt T, Furman J M. Psychiatric aspects of dizziness and imbalance. In: Bronstein AM, Brandt T, Woolacott MH, et al, editors. Clinical disorders of balance, posture and gait. London: Arnold, 2004245–281.
- 37.Cevette M J, Puetz B, Marion M S.et al A physiologic performance on dynamic posturography. Otolaryngol Head Neck Surg 1995112676–688. [DOI] [PubMed] [Google Scholar]
- 38.FitzGerald J E, Birchall J P, Murray A. Identification of non‐organic instability by sway magnetometry. Br J Audiol 199731275–282. [DOI] [PubMed] [Google Scholar]
- 39.Anderson G, Hajman J, Talianzadek R.et al Dual task study of cognitive and postural interference in patients with vestibular disorders. Otol Neurootol 200324289–293. [DOI] [PubMed] [Google Scholar]
- 40.Eckhardt‐Henn A, Dieterich M. Psychiatric Disorders in Neurootologic Patients. Neurologic Clinics 200523731–749. [DOI] [PubMed] [Google Scholar]
- 41.Baloh R W, Honrubia V. Laboratory examination of the vestibular system. In: Baloh, RW, Honrubia V, editors. Clinical neurophysiology of the vestibular system, 3rd edition. New York: Oxford University Press, 2001155–199.
- 42.Kamei T, Kornhuber H H. Spontaneous and head shaking nystagmus in normals and in patients with central lesions. Can J Otolaryngol 19743372–374. [Google Scholar]
- 43.Sullivan M, Clark M R, Katon W J.et al Psychiatric and otologic diagnoses in patients complaining of dizziness. Arch Intern Med 19931531479–1484. [PubMed] [Google Scholar]
- 44.Swinson R P, Cox B J, Rutka J.et al Otoneurological functioning in panic disorder patients with prominent dizziness. Compr Psychiatry 199334127–129. [DOI] [PubMed] [Google Scholar]
- 45.Pollak L, Klein C, Stryjer R.et al Phobic postural vertigo: a new proposed entity. Isr Med Assoc J 20035720–723. [PubMed] [Google Scholar]
- 46.Staab J P, Ruckenstein M J. Which comes first? Psychogenic dizziness versus otogenic anxiety. Laryngoscope 20031131714–1718. [DOI] [PubMed] [Google Scholar]