Abstract
Background
Non‐missile traumatic brain injury (nmTBI) without macroscopically detectable lesions often results in cognitive impairments that negatively affect daily life.
Aim
To identify abnormal white matter projections in patients with nmTBI with cognitive impairments using diffusion tensor magnetic resonance imaging (DTI).
Methods
DTI scans of healthy controls were compared with those of 23 patients with nmTBI who manifested cognitive impairments but no obvious neuroradiological lesions. DTI was comprised of fractional anisotropy analysis, which included voxel‐based analysis and confirmatory study using regions of interest (ROI) techniques, and magnetic resonance tractography of the corpus callosum and fornix.
Results
A decline in fractional anisotropy around the genu, stem and splenium of the corpus callosum was shown by voxel‐based analysis. Fractional anisotropy values of the genu (0.47), stem (0.48), and splenium of the corpus callosum (0.52), and the column of the fornix (0.51) were lower in patients with nmTBI than in healthy controls (0.58, 0.61, 0.62 and 0.61, respectively) according to the confirmatory study of ROIs. The white matter architecture in the corpus callosum and fornix of patients with nmTBI were seen to be coarser than in the controls in the individual magnetic resonance tractography.
Conclusions
Disruption of the corpus callosum and fornix in patients with nmTBI without macroscopically detectable lesions is shown. DTI is sensitive enough to detect abnormal neural fibres related to cognitive dysfunction after nmTBI.
Cognitive and vocational sequelae are common complications after non‐missile traumatic brain injury (nmTBI) without obvious neuroradiological lesions.1,2 They may present as memory disturbance, impairments in multitask execution and loss of self‐awareness.3 These symptoms have been attributed to diffuse brain injury and the diffuse loss of white matter or neural networks in the brain.4,5,6 Currently no accurate method is available for diagnosing and assessing the distribution and severity of diffuse axonal injury. As computed tomography and magnetic resonance imaging (MRI) findings underestimate the extent of diffuse axonal injury and correlate poorly with the final neuropsychological outcome,7,8 this dysfunction tends to be clinically underdiagnosed or overlooked. Indirect evidence for loss of functional connectivity after nmTBI has been provided by both morphometric and functional neuroimaging studies. Morphometric analysis of nmTBI has shown the relationship between atrophy of the corpus callosum and fornix and the neuropsychological outcome.9 Most functional neuroimaging studies conducted after nmTBI have shown that cognitive and behavioural disorders are correlated, with some degree of secondary hypometabolism or hypoperfusion in regions of the cortex.5 To date, however, there has been no direct in vivo demonstration of structural disconnections without macroscopically detectable lesions in patients with nmTBI.
Diffusion tensor magnetic resonance imaging (DTI), which measures diffusion anisotropy in vivo, is a promising method for the non‐invasive detection of the degree of fibre damage in various disease processes affecting the white matter.10,11 In biological systems, the diffusional motion of water is impeded by tissue structures, such as cell membranes, myelin sheaths, intracellular microtubules and associated proteins. Motion parallel to axons or myelin sheaths is inhibited to a lesser degree than perpendicular motion, a phenomenon known as diffusion anisotropy.12 Fractional anisotropy was applied to evaluation of post‐traumatic diffuse axonal injury13 and its clinical usefulness described. In a previous study,14 fractional anisotropy score in the acute stage as an index of injury to white matter showed promise in predicting outcome in patients with traumatic brain injury, by using the regions of interest (ROIs) techniques. MRI voxel‐based analysis, a statistical normalising method, has been developed to reduce interindividual variability and to evaluate the whole brain objectively.15,16,17 We investigated the regions in the whole brain that are commonly injured in patients having nmTBI with cognitive impairments but no macroscopic lesions, using voxel‐based analysis of fractional anisotropy, referred to as diffusion anisotropy. The advent of DTI has allowed inter‐regional fibre tracking, called magnetic resonance tractography, which reconstructs the three‐dimensional trajectories of white matter tracts.11,18,19 We also investigated whether magnetic resonance tractography sensitively recognises degeneration of the corpus callosum and fornix in individual patients with nmTBI.
Methods
Patient population
We studied 23 patients in the chronic stage after they had severe nmTBI and had recovered from coma. All of them had sustained a high‐velocity, high‐impact injury in a motor vehicle accident. Table 1 shows the clinical features and results of neuropsychological examinations of patients involved in the motor vehicle accident. The study population was selected from 51 consecutive patients with nmTBI who were entered into the rehabilitation programme of Chubu Medical Center, Gifu, Japan. Patients with severe language or attention deficits that prevented neuropsychological testing were excluded from the study. Patients with physical deficits or neuroradiologically detectable lesions larger than 1.6 cm3, such as contusions, haematomas or infarcts on the high spatial resolution T1‐weighted image MRI collected at the time of the study, were also excluded. In a previous paper, Tomaiuolo et al16 defined patients with neuroradiologically detectable lesions smaller than 1.6 cm3 as patients with nmTBI without macroscopically detectable lesions, so we adopted 1.6 cm3 as a cut‐off point in our study. Neuropsychological testing was carried out within 2 weeks of obtaining MRI scans. For overall estimation of their global intellectual, mnemonic performance and attention, we administered the Wechsler Adult Intelligence Scale—Revised (WAIS—R) test, the Mini‐Mental State Examination (MMSE), the Wechsler Memory Scale—Revised (WSM—R) test and the Paced Auditory Serial Addition Test (PASAT); all tests were the Japanese language version. The controls were 23 healthy participants matched for sex and age. Neither the patients nor the controls had a history of neurological or psychiatric disorders. All participants gave prior written informed consent. The research committee of Kizawa Memorial Hospital Foundation approved the protocol.
Table 1 Demographic data on patients with traumatic brain injury and healthy controls.
| Factor | Patients | Controls |
|---|---|---|
| Mean age (years) | 27.43 (12.09) | 28.23 (11.90) |
| Sex | ||
| Male | 19 | 19 |
| Female | 4 | 4 |
| Mean duration of impaired consciousness (days) | 7.2 (8.6) | — |
| Mean interval between injury and MRI (months) | 14.16 (16.2) | — |
| Wechsler Adult Intelligence Scale—Revised | ||
| Full‐scale Intelligence Quotient | 80.4 (11.2) | 104.2 (14.1) |
| Verbal Intelligence Quotient | 84.6 (12.2) | 103.6 (12.9) |
| Performance Intelligence Quotient | 78.0 (13.5) | 105.2 (15.9) |
| Mini‐Mental State Examination | 26.3 (4.5) | 29.7 (0.8) |
| Wechsler Memory Scale—Revised | ||
| General memory | 71.7 (14.6) | 106.3 (12.1) |
| Delayed memory | 66.5 (13.2) | 103.7 (9.8) |
| Visual memory | 71.0 (14.0) | 102.1 (7.5) |
| Attention | 85.3 (16.3) | 107.3 (7.2) |
| Paced Auditory Serial Addition Test | 34.5 (10.6) | 46 (6.2) |
MRI scanning protocol
All participants underwent examinations with a 1.5T Signa MRI system (GE Medical Systems, Milwaukee, Wisconsin, USA). The rapid‐gradient echo T1‐weighted images were obtained for judging the nmTBI. We used a single‐shot spin‐echo planar sequence (TR/TE, 10 000/79 ms; slice thickness, 3 mm; field of view, 25 cm2; number of experiments, 4; pixel matrix, 128×128) for diffusion tensor analysis. Diffusion gradients (b = 1000 s/mm2) were always applied on two axes simultaneously around the 180 pulses. Diffusion properties were measured along six non‐collinear directions. Diffusion‐weighted magnetic resonance images were transferred to a workstation supplied by the manufacturer (Advantage Workstation, GE Medical Systems); structural distortion induced by large diffusion gradients was corrected on the basis of T2‐weighted echo–planar images (b = 0 s/mm2). The six elements of the diffusion tensor were estimated in each voxel, assuming a monoexponential relationship between signal intensity and the b matrix. The eigenvectors and eigenvalues (λ1>λ2>λ3) of the diffusion tensor were determined by using multivariate analysis. Fractional anisotropy maps were generated on a voxel‐by‐voxel basis as follows:
FA=√3/2×√[(λ1−MD)2+(λ2−MD)2+(λ3−MD)2]/√(λ12+λ22+λ32).
Fractional anisotropy template creation, spatial normalisation and voxel‐based analysis with fractional anisotropy map
Spatial normalisation is an essential preprocessing step in voxel‐based analysis.19 The contrast of the fractional anisotropy map is different from that of T1‐weighted and T2‐weighted and other template images in the statistical parametric mapping software package (SPM99; Wellcome, Department of Cognitive Neurology, London, UK) and it is necessary to make a fractional anisotropy template to normalise the individual fractional anisotropy maps correctly. We created a fractional anisotropy template from all participants (patients and controls). Individual fractional anisotropy maps were made by the Advantage Workstation (GE Medical Systems) and transferred to a Windows with SPM99 running on MatlabV.5.3 (Mathworks, Natic, Massachusetts, USA). T2‐weighted echo–planar images of all participants were transformed to the T2‐weighted template. The parameter of the transformation was applied to the fractional anisotropy maps. Normalised individual fractional anisotropy maps were smoothed with an 8‐mm full‐width at half‐maximum isotropic gaussian kernel. The fractional anisotropy template was created for all participants, and individual fractional anisotropy maps of controls and patients were normalised with the original fractional anisotropy template. The normalised fractional anisotropy images were smoothed with an 8‐mm full‐width at half‐maximum isotropic gaussian kernel.
Once the images had been spatially normalised and smoothed, group comparisons (two samples t test) were applied to calculate the statistical significance between the control and patient group on SPM99. The statistical parametric maps for comparison of the patients with controls and for covariate effects of MMSE, WAIS—R, WMS—R and PASAT by using regression modelling were thresholded at a value of corrected p<0.05 in voxel level. The statistical map was overlapped on the fractional anisotropy template.
Confirmatory study of ROIs with fractional anisotropy maps and magnetic resonance tractography
Confirmatory analysis was subsequently conducted using ROIs. Several round ROIs with a diameter of 2 mm were placed on individual fractional anisotropy maps in pathways that were identified by voxel‐based analysis as having lower fractional anisotropy values in our nmTBI sample of the whole brain. This included the corpus callosum and fornix. Control ROIs were placed bilaterally in the corona radiata and centrum semiovale. The mean values of fractional anisotropy from several round ROIs for every neuroanatomical region were used as individual values. A non‐parametric test (Mann–Whitney U test) was used to examine group differences. A p = 0.01 (two tailed) was chosen as the significance threshold. Magnetic resonance tractography was made with diffusion tensor visualisation software.19 For tractography of the corpus callosum, seed volumes were located from the genu to the splenium through the body on reconstructed mid‐sagittal images. For tractography of the fornix, seed volumes were located in the column of the fornix on reconstructed coronal images. The fractional anisotropy value for stop criteria was 0.18. Tractographic results were overlaid on T2‐weighted images.
Results
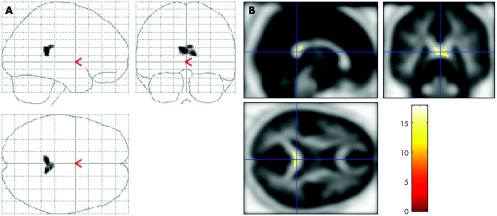
Figure 1 shows the voxel‐based analysis of our fractional anisotropy data with the fractional anisotropy template from all participants (controls and patients). An SPM in the three orthogonal maximum‐intensity projections showed voxels with lower values of fractional anisotropy in the patients with nmTBI than in the controls. A marked decrease in fractional anisotropy values was found in the corpus callosum in the group with nmTBI. In other regions, no marked decrease or increase was noted.
Figure 1 (A) Voxel‐based analysis of fractional anisotropy data with the fractional anisotropy template for all participants. Statistical parametric map in the three orthogonal maximum‐intensity projections show voxels with lower fractional anisotropy values in patients with traumatic brain injury than in controls. Peak coordinates are (x, y, z (mm)) = (−14, 14, 26), (2, 5, 24), (12, 8, 28) (corpus callosum, k = 746; Z score = 5.98, 5.73, 5.55; uncorrected p<0.001), (10, −34, 22), (−2, −32, 22), (−14, −38, 30) (corpus callosum, k = 94; Z score = 5.16, 5.15, 5.14; uncorrected p<0.001). (B) Voxels with a marked decrease in fractional anisotropy values in patients with traumatic brain injury compared with controls. A considerable decrease in fractional anisotropy values was found in the group with traumatic brain injury. The results of voxel‐based analysis are rendered on orthogonal slices of the fractional anisotropy map.
The relationship between cognitive scores and fractional anisotropy value was investigated in patients with nmTBI. Figure 2 shows the positive correlation effects of cognitive score (MMSE). A notable correlation was found in the splenium of the corpus callosum. The SPM for covariate effects of the other cognitive scores using regression modelling were also thresholded at a value of corrected p<0.05 in voxel level and at an extent of 60 voxels. No significant correlation effect of WAIS—R, WMS—R and PASAT was observed under this condition.
Figure 2 (A) Results of voxel‐based analysis for covariate effects of Mini‐Mental State Examination (MMSE). Statistical parametric map in the three orthogonal maximum‐intensity projections, showing voxels with the positive correlation effects of MMSE. Peak coordinates are (x, y, z (mm)) = (−2, −38, 16), (10, −40, 12), (5, −34, 20) (splenium of the corpus callosum, k = 137; Z score = 6.01, 5.88, 5.78; uncorrected p<0.001). (B) Voxels with a marked correlation between cognitive scores (MMSE) and fractional anisotropy. A considerable correlation was found in the splenium of the corpus callosum. The results of voxel‐based analysis are rendered on orthogonal slices of the fractional anisotropy map.
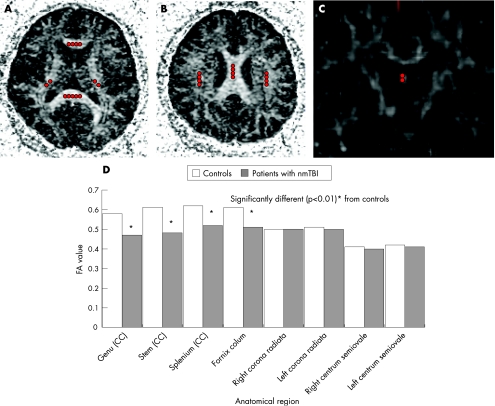
Several round ROIs with a diameter of 2 mm were placed in the corpus callosum, the column of the fornix, the corona radiata bilaterally and the centrum semiovale bilaterally on individual fractional anisotropy maps (figs 3A–C). The mean values of fractional anisotropy from several round ROIs were used. Examination of group differences by study of ROIs confirmed the marked differences in fractional anisotropy, noted in the voxel‐based analysis. We found considerable differences between the group with nmTBI group and controls in the fractional anisotropy values of the genu (p = 0.0008), stem (p = 0.006) and splenium of the corpus callosum (p = 0.009), and the column of the fornix (p = 0.009; fig 3D). Average values generated from the ROI placed in the corticospinal tract did not differ between the two groups.
Figure 3 (A–C) Axial and coronal fractional anisotropy (FA) maps. Several circular regions of interest with a diameter of 2 mm are placed in the corpus callosum (CC; the genu, stem and splenium), column of the fornix, corona radiata and centrum semiovale. (D) FA values in the CC, fornix, corona radiata and centrum semiovale of patients with traumatic brain injury. nmTBI, non‐missile traumatic brain injury.
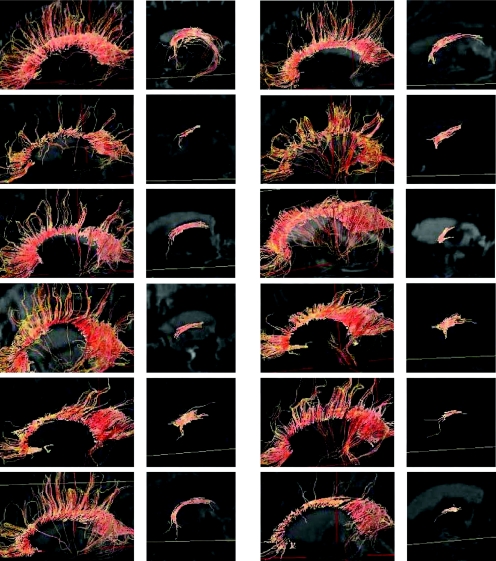
Figure 4 compares individual magnetic resonance tractography of the corpus callosum and the fornix from some nmTBI cases with controls. In cases with nmTBI, the tracking lines through the genu and the splenium of the corpus callosum were different from those in controls, with the connecting fibres not reaching the cortex. The volume and the connecting fibres from the splenium in patients with nmTBI were relatively retained. Compared with controls, the tracking lines through the column of the fornix in patients with nmTBI did not pass along the fimbria of the hippocampus, although tractography around the mamillary body to the column of the fornix was relatively retained.
Figure 4 Lateral view for the tractography of the corpus callosum (left column) and fornix (right column) in a control (left upper) and in 11 patients with traumatic brain injury. The tractographs are overlaid on T2‐weighted MRI.
Discussion
Our results suggest that DTI was able to objectively show abnormalities in patients with nmTBI with cognitive impairments but without macroscopically detectable lesions. To our knowledge, this is the first report of white matter disruption of the corpus callosum and fornix to evaluate nmTBI without macroscopically detectable lesions by using DTI. Voxel‐based fractional anisotropy analysis and tractography study objectively showed the vulnerability of the corpus callosum and fornix in patients with nmTBI. The parasagittal subcortical white matter, internal capsules, cerebellar folia dorsal to the dentate nuclei and brain stem, but not corpus callosum and fornix, are susceptible to diffuse axonal injury.20 Our study showed the specific vulnerability of the corpus callosum and fornix, because our patients with nmTBI had cognitive impairments but no physical problems. The corpus callosum and fornix are thought to be the structures at the core of neural networks in cognition and memory. Changes in the anterior white matter, including the corpus callosum, were strongly related to age‐related cognitive decline.21,22 The fornix is the major limbic white matter pathway interconnecting the hippocampus and the mamillary bodies. The limbic circuitry of the hippocampus–fornix–mamillary body interaction has been the focus of extensive research on memory function. Damage to any of these limbic structures results in various memory disorders.23
Limitations exist in assessing fractional anisotropy value by ROI techniques, especially in the case of very small ROI with a diameter of 2 mm. ROI techniques depend either on subjective assessment or on the relatively arbitrary size, shape and placement of the ROIs. As a result, some areas of the brain may not be explored. SPM analysis is an alternative voxel‐by‐voxel analysis method that can avoid subjectivities. In this study, a marked decrease in values of fractional anisotropy in the column of the fornix was not obvious with SPM. The SPM may not have been successful because of the small size of the fornix. Its location in stereotactic space may have been displaced between subjects because of normalisation errors and smeared out during smoothing with a gaussian low‐pass filter of an 8‐mm full‐width at half‐maximum, making differences harder to detect. Because it was relatively easy to detect on the native fractional anisotropy images, the manual ROI values, especially in the small size, may be more accurate, resulting in greater statistical power.
In this study, a marked correlation was found in the splenium of the corpus callosum between MMSE and fractional anisotropy value. No significant correlation effects of WMS—R and PASAT were, however, detected. Although the condition with threshold at a value of corrected p<0.05 in voxel level and at an extent of 60 voxels may cause these results, a positive correlation was shown only in the MMSE may be because of characteristic differences between the MMSE and the others. The MMSE reflects general cognitive function; the others, however, reflect specific cognitive function.
Although a major number of patients with diffuse brain damage and cognitive impairments do not show obvious lesions on conventional neuroimaging, quantitative brain MRI studies conducted at least 6 weeks after injury have shown that moderate to severe traumatic brain injury results in a decrease in the volume of the hippocampus, fornix and corpus callosum.24,25,26 These reports point to the vulnerability of the corpus callosum and fornix in patients with nmTBI and the relationship between atrophy and cognitive dysfunction. The results of these morphometric studies and our investigation on abnormalities of the corpus callosum and fornix are consistent. White matter abnormalities in patients with nmTBI can be detected earlier with DTI than with the morphometric methods, because DTI can presume the structure of the white matter, whereas morphometrics may detect secondary, atrophic changes subsequent to primary damage. The altered state of the white matter resulting from acute traumatic brain injury may, however, affect diffusion tensor anisotropy measurements. Oedematous tracts may lose some anisotropy, but retain enough directional organisation to remain identifiable on directional DTI.27 White matter tracts may be destroyed or disruptured to the point where directional organisation and, consequently, diffusion anisotropy is lost completely. In our study, magnetic resonance tractography of the corpus callosum and fornix disclosed abnormalities in the tracking lines of the individual patients with nmTBI . The magnetic resonance tractography was symbolic and demonstrative of the neural network and may be useful for individual evaluation of patients with nmTBI. The definition of magnetic resonance tractography as a clinical method has, however, not been established. The technical limitations of magnetic resonance tractography must not be overlooked.28 Studies are under way in our hospital to identify the optimal timing of DTI to identify accurately the presence of white matter abnormalities after nmTBI.
In this study, all participants underwent DTI with the 1.5T MRI system. Although a direct comparison of 3.0T and 1.5T with regard to magnetic resonance tractography has not been reported, the 3.0T MRI system has a higher signal‐to‐noise ratio than 1.5T. Thin slice images can be obtained with 3.0T owing to a higher signal‐to‐noise ratio, so z‐axis resolution is improved. Therefore, magnetic resonance tractography may be more effective in z axis with 3.0T than with 1.5T. The 3.0T system, however, has a higher susceptibility artefact in DTI. It will be necessary to examine the clinical comparison of 3.0T imaging and 1.5T imaging with regard to magnetic resonance tractography furthermore.
We will apply our findings to each patient with traumatic brain injury in the acute or subacute stage, to obtain an objective index of reliable diagnosis, evaluation, estimation and treatment of cognitive impairments.
Acknowledgements
This study was supported in part by the Gifu Prefecture Brain Research Foundation and a medical research grant on traffic accidents from the General Insurance Association of Japan. We thank Hirata Naoki (General Electric Yokogawa Medical Systems, Tokyo, Japan) for technical assistance.
Abbreviations
DTI - diffusion tensor magnetic resonance imaging
MMSE - Mini‐Mental State Examination
nmTBI - non‐missile traumatic brain injury, PASAT, Paced Auditory Serial Addition Test
ROI - region of interest
SPM - statistical parametric mapping
WAIS—R - Wechsler Adult Intelligence Scale—Revised
WMS—R - Wechsler Memory Scale—Revised
Footnotes
Competing interests: None declared.
References
- 1.Levin H S, Gary H E, Jr, Eisenberg H M.et al Neurobehavioral outcome 1 year after severe head injury: experience of the Traumatic Coma Data Bank. J Neurosurg 199073699–709. [DOI] [PubMed] [Google Scholar]
- 2.Levin H S, Williams D H, Eisenberg H M.et al Serial MRI and neurobehavioral findings after mild to moderate closed head injury. J Neurol Neurosurg Psychiatry 199255255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin H S. Neurobehavioral recovery. J Neurotrauma 19929(Suppl)359–373. [PubMed] [Google Scholar]
- 4.Gennarelli T A, Thibault L E, Adams J H.et al Diffusion axonal injury and traumatic coma in the primate. Ann Neurol 198212564–574. [DOI] [PubMed] [Google Scholar]
- 5.Fontaine A, Azouvi P, Remy P.et al Functional anatomy of neuropsychological deficits after severe traumatic brain injury. Neurology 1999531963–1968. [DOI] [PubMed] [Google Scholar]
- 6.Vilkki J. Cognitive flexibility and mental programming after closed head injuries and anterior or posterior cerebral excisions. Neuropsychologia 199230807–814. [DOI] [PubMed] [Google Scholar]
- 7.Kelly A B, Zimmerman R D, Snow R B.et al Head trauma: comparison of MR and CT‐experience in 100 patients. Am J Neuroradiol 19889699–708. [PMC free article] [PubMed] [Google Scholar]
- 8.Levi L, Guilburd J N, Lemberger A.et al Diffuse axonal injury: analysis of 100 patients with radiological signs. Neurosurgery 199027429–432. [PubMed] [Google Scholar]
- 9.Gale S, Johnson S C, Bigler E D.et al Nonspecific white matter degeneration following traumatic brain injury. J Int Neuropsychol Soc 1995117–28. [DOI] [PubMed] [Google Scholar]
- 10.Ellis C M, Simmons A, Jones D K.et al Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 1999531051–1058. [DOI] [PubMed] [Google Scholar]
- 11.Konishi J, Yamada K, Kizu O.et al MR tractography for the evaluation of functional recovery from lenticulostriate infarcts. Neurology 200564108–113. [DOI] [PubMed] [Google Scholar]
- 12.Moseley M E, Cohen Y, Kucharczyk J.et al Diffusion tensor MR imaging of anisotropic water diffusion in cat central nervous system. Radiology 1990176439–445. [DOI] [PubMed] [Google Scholar]
- 13.Arfanakis K, Haughton V M, Carew J D.et al Diffusion tensor MR imaging in diffuse axonal injury. Am J Neuroradiol 200223794–802. [PMC free article] [PubMed] [Google Scholar]
- 14.Ptak T, Sheridan R L, Rhea J T.et al Cerebral fractional anisotropy score in trauma patients: a new indicator of white matter injury after trauma. Am J Roentgenol 20031811401–1407. [DOI] [PubMed] [Google Scholar]
- 15.Good C D, Johnsrude I S, Ashburner J.et al A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 20011421–36. [DOI] [PubMed] [Google Scholar]
- 16.Tomaiuolo F, Carlesimo G A, Paola D I.et al Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non‐missile traumatic brain injury without macroscopically detectable lesions: a T1 weighted MRI study. J Neurol Neurosurg Psychiatry 2004751314–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abe O, Yamada H, Masutani Y.et al Amyotrophic lateral sclerosis: diffusion tensor tractography and voxel‐based analysis. NMR Biomed 200417411–416. [DOI] [PubMed] [Google Scholar]
- 18.Mori S, Kaufmann W E, Pearlson G D.et al In vivo visualization of human neural pathways by magnetic resonance imaging. Ann Neurol 200047412–414. [PubMed] [Google Scholar]
- 19.Masutani Y, Aoki S, Abe O.et al MR diffusion tensor imaging: recent advance and new techniques for diffusion tensor visualization. Eur J Radiol 20034653–66. [DOI] [PubMed] [Google Scholar]
- 20.Adams J H, Graham D I, Murray L S.et al Diffuse axonal injury due to non‐missile head injury in humans: an analysis of 45 cases. Ann Neurol 198212557–563. [DOI] [PubMed] [Google Scholar]
- 21.O'Sullivan M, Jones D K, Summers P E.et al Evidence for cortical “disconnection” as a mechanism of age‐related cognitive decline. Neurology 200157632–638. [DOI] [PubMed] [Google Scholar]
- 22.Head D, Buckner R L, Shimony J S.et al Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex 200414410–423. [DOI] [PubMed] [Google Scholar]
- 23.Garcia‐Bengochea F, Friedman W A. Persistent memory loss following section of the anterior fornix in humans. A historical review. Surg Neurol 198727361–364. [DOI] [PubMed] [Google Scholar]
- 24.Gale S D, Burr R B, Bigler E D.et al Fornix degeneration and memory in traumatic brain injury. Brain Res Bull 199332345–349. [DOI] [PubMed] [Google Scholar]
- 25.Levin H S, William D H, Valastro M.et al Corpus callosum atrophy following closed head injury: detection with magnetic resonance imaging. J Neurosurg 19907377–81. [DOI] [PubMed] [Google Scholar]
- 26. Tate DF, Bigler , eds. Fornix and hippocampal atrophy in traumatic brain injury. Learning Memory 20007442–446. [DOI] [PubMed] [Google Scholar]
- 27.Witwer B P, Moftakhar R, Hasan K M.et al Diffusion‐tensor imaging of white matter tracts in patients with cerebral neoplasm. J Neurosurg 200297568–575. [DOI] [PubMed] [Google Scholar]
- 28.Tuch D S, Reese T G, Wiegell M R.et al High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med 200248577–582. [DOI] [PubMed] [Google Scholar]