Abstract
Background
The memory deficits in patients with temporal lobe epilepsy (TLE) are associated with epileptogenic lesions of the temporal lobes, especially hippocampal sclerosis. Memory deficits have been extensively studied in TLE, but the presence of pre‐existing temporal lobe abnormality has confounded studies on the relationship between memory dysfunction and seizure activity. Idiopathic generalised epilepsy (IGE) is characterised by primary generalised seizures and is found to occur in the absence of any macroscopic brain abnormalities. IGE is therefore ideal for investigations on the effects of seizure activity on memory and cognition.
Aim and methods
Magnetic resonance spectroscopy (MRS) and neuropsychological testing were used to investigate the relationship between epileptic seizures, memory performance and neuronal dysfunction in the temporal lobes of a group of patients with IGE. 30 patients and 15 healthy controls participated in the study.
Results
Patients with IGE were found to perform worse than controls on tests of speed of information processing, general cognitive performance and a range of memory tests, including face recognition, word recognition, verbal recall and complex figure recall. The performance of the patient group on the visual recognition and verbal recall sections of the Doors and People Test was found to correlate with MRS ratios of N‐acetyl aspartate:choline and N‐acetyl aspartate:creatine in the temporal lobes.
Conclusion
This result supports the hypothesis that memory deficits in epilepsy may be due to neuronal dysfunction secondary to epileptic activity itself in the absence of any macroscopic lesions in the temporal lobes.
Idiopathic generalised epilepsy (IGE) is a group of epilepsy syndromes characterised by primary generalised seizures,1 including absence seizures, myoclonic seizures and generalised tonic–clonic seizures (GTCS). Epileptic activity in generalised seizures is principally bilateral, synchronous and symmetrical.2 Seven types of IGE are recognised by the International League Against Epilepsy2 and their classification is based on age at onset and the pattern of occurrence of each of the types of generalised seizures. An essential characteristic of all these syndromes is that the brain is macroscopically normal.1
Cognitive decline in IGE, especially memory deficits, has received scant attention in the literature. In contrast, memory deficits in temporal lobe epilepsy (TLE) have been extensively studied. Memory deficits in TLE have been found in all the major domains and the degree of impairment correlates with quality of life.3 Patients are reported to perform worse than controls on verbal memory,4 visual memory,5 episodic memory,6 and memory for facts and words.7 Much of the literature shows an interaction between laterality of the lesion and the type of memory deficit.8 If the epileptic focus is in the left hemisphere, language and verbal deficits predominate slightly. A right‐sided focus, however, leads to a slight predominance of non‐verbal deficits.
The epileptogenic lesion in the temporal lobes in TLE is commonly hippocampal sclerosis.6,9 The temporal lobe, and especially the hippocampus, is crucial for normal memory function,10,11 and abnormality in these structures is associated with memory deficits12 in TLE. Cognitive impairments in TLE are probably compounded by the disruption of coherent neuronal discharges by epileptic activity. Patients with TLE show memory deficits that are associated with the function of the unaffected temporal lobe.12 This suggests that epileptic activity in focal epilepsy can cause widespread disruption of neuronal activity remote from the epileptogenic lesion.
Magnetic resonance proton spectroscopy has been used in neurological disease to characterise gross structural lesions and to detect neuronal dysfunction and subtle changes in neuronal density in patients whose brains are macroscopically normal.13,14,15,16,17,18 Although its function is unknown, N‐acetyl aspartate (NA) is found only in neurones19 and decreases in N‐acetyl aspartate levels are seen in diseases of the nervous system.20 In contrast, creatine (Cr) is a ubiquitous compound found in both neurones and glial cells.13 Its level remains relatively stable even during disease and therefore magnetic resonance spectroscopy (MRS) results are often expressed as a ratio of NA:Cr and this value is taken to reflect neuronal dysfunction or changes in neuronal density. Choline (Cho) is bound to cell membranes, myelin and complex brain lipids. It is also used as a marker for background cellular density and so the ratio NA:Cho is used as another indicator of neuronal dysfunction.13
Our experience of patients with IGE is that they often report memory deficits such as poor performance on recall of everyday items, including telephone numbers, domestic chores, messages to pass on and even poor recognition of familiar faces. Although subtle histological21, MRI22 and spectroscopic abnormalities have been demonstrated23 in IGE there is no gross structural lesion, in contrast to TLE. Therefore, IGE is a good model to test the hypothesis that epileptic activity itself causes dysfunction in the temporal lobes that leads to memory impairment.
Methods
Selection of participants
RAG and SJLH recruited patients with IGE from the Epilepsy Clinic at the Royal Hallamshire Hospital, Sheffield, UK. Diagnosis was based on clinical and electroencephalographic (EEG) criteria.2 Healthy controls were recruited by means of posters placed around the Royal Hallamshire Hospital and from a database of volunteers held by the Academic Neurology Unit at The University of Sheffield. Healthy controls did not have any neurological illness and had never sustained a major head injury. In all, 30 patients and 15 healthy controls took part in the study and underwent neuropsychological testing and magnetic resonance proton spectroscopy. All participants gave written informed consent and the South Sheffield Research Ethics Committee (Ethics number 98/269) approved the study. As no previous study correlating neuropsychological variables with MRS variables in patients with IGE has been published, the sample size was chosen to be similar to that of other studies that have used correlational analysis of magnetic resonance data and cognitive variables.24
Epilepsy assessment
The Chalfont Severity of Seizures Scale25 was used to assess the effect of each type of seizure on the patient's life. Total lifetime number of each type of seizure was estimated by the patient in consultation with JMD, who had access to the patient's medical records. The patients were also asked “Do you have problems with your memory?” Their response was recorded as yes or no. Current medication was recorded.
Neuropsychology
None of the patients had experienced a GTCS during the 24 h before testing. The tests assessed memory performance (Doors and People (D&P),26 Story Recall, Complex Figure Recall, Face Recognition, Word Recognition), IQ (National Adult Reading Test (NART),27 Wechsler Adult Intelligence Scale—Revised (WAIS‐R)28) and speed of information processing (Speed and Capacity of Language Processing (SCOLP)29). Some of the tests were commercial tests with published normative data and some were bespoke tests designed in the Academic Neurology Unit at The University of Sheffield.
MRI
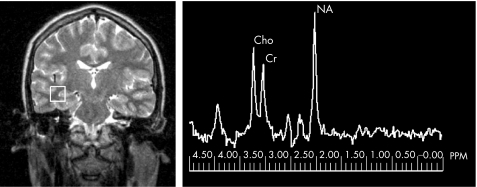
Each of the participants underwent MRI that included magnetic resonance proton spectroscopy. The entire scanning session took about 1 h. MRI was carried out at 1.5 T (Eclipse, Philips Medical Systems, Cleveland, Ohio, USA). Single‐voxel spectra were obtained for each participant, by using a point‐resolved spectroscopy technique (echo time 135 ms; repetition time 1600 ms; 256 averages), predominantly from the white matter of both temporal lobes but also including some deep and neocortical grey matter. A Gauss–Lorentz fit was carried out and the areas under the three prominent resonances were calculated: N‐acetyl aspartate groups at 2.02 ppm; total creatine at 3.0 ppm and compounds containing choline at 3.2 ppm. Figure 1 shows the position of a typical voxel and a typical spectrum. Results were expressed in terms of ratios: NA:Cho, NA:Cr and Cho:Cr. The images were also reviewed by an experienced neuroradiologist (PDG) to exclude significant unexpected pathology.
Figure 1 Data from a patient with idiopathic generalised epilepsy. (Left) A spectroscopic voxel located in the temporal lobe white matter. (Right) The resultant spectrum. Cho, choline; Cr, creatine; NA, N‐acetyl aspartate; PPM, parts per million.
Three results are reported for each of the ratios: left, right and summary. Left and right refer to data from voxels in the left and right temporal lobes, respectively. For participants with data from both voxels, the summary value is the mean of the two voxels. In cases where data are missing from one voxel, the data from the other voxel are reported as the summary value.
Statistical analysis
Data were statistically analysed with SPSS V.11.0.1 on a personal computer running Microsoft Windows XP. Data were assessed for normality by using the Kolmogorov–Smirnov statistic as well as visual inspection of histograms, normal Q–Q plots and detrended normal Q–Q plots. Parametric and non‐parametric statistics were applied where appropriate. The following tests were used for group comparisons: independent samples t test, paired samples t test, Mann–Whitney U test and Wilcoxon's signed rank test. To investigate the relationships between variables, Spearman's ρ (rs), partial correlation and scatter graphs were used. We considered p⩽0.05 to indicate significance and have presented uncorrected p values for each of the analyses.30
Results
Participants and handling of missing data
One of the patients, subsequently diagnosed as having cortical dysplasia, was excluded from the study. Therefore, results from 29 patients (12 men and 17 women) and 15 healthy controls (6 men and 9 women) are shown. Of the 29 patients, 5 fulfilled the criteria for juvenile myoclonic epilepsy; 1 had GTCS on awakening; and the rest had IGE of uncertain syndromic classification with GTCS with or without typical absences associated with generalised spike wave discharges on the EEG. An independent samples t test showed no significant difference between the mean age of the patients (mean 30 (SD 12.6)) and the healthy controls (mean 34 (SD 13.9) years; t (42) = −0.923; p = 0.361).
All the patients except two were taking epilepsy drugs. Eight of these patients were taking monotherapy and the rest were taking at least two epilepsy drugs. Table 1 shows the drugs and doses taken.
Table 1 Drugs (mg) taken at the time of testing.
| Valproate | Lamotrigine | Ethosux | Carbamaz | Topiramate | |
|---|---|---|---|---|---|
| Number of patients | 17 | 17 | 3 | 2 | 4 |
| Median | 1500 | 225 | 800 | 1300 | 200 |
| Minimum | 200 | 50 | 800 | 1000 | 75 |
| Maximum | 3000 | 600 | 800 | 1600 | 300 |
| Primidone | Levetir | Sertraline | Ranitidine | Phenobar | |
|---|---|---|---|---|---|
| Number of patients | 2 | 5 | 1 | 1 | 1 |
| Median | 1000 | 1500 | 150 | 150 | 90 |
| Minimum | 1000 | 750 | 150 | 150 | 90 |
| Maximum | 1000 | 3000 | 150 | 150 | 90 |
Carbamaz, carbamazepine; ethosux, ethosuxamide; levetir, levetiracetam; phenobar, phenobarbitone; Valproate, sodium valproate.
Three of the patients did not attend MRI and some of the MRS scans were affected by poor tolerance of the procedure, poor water suppression or inadequate shim such that there were unusable data from either one or both of the spectroscopy voxels. This was the case for 12 patients and four healthy controls. The neuropsychological tests were completed by all the participants with the following exceptions: (a) word recognition—2 patients, (b) SCOLP—1 patient and 1 healthy control and (c) D&P—3 healthy controls. Missing data were excluded from statistical analyses on a test‐by‐test basis.
General measures
Table 2 shows summary statistics for epileptic activity in the patients. In all, 25 of the 29 (86%) patients answered yes to the question “Do you have problems with your memory?”, whereas none of the healthy controls reported a memory deficit.
Table 2 Summary data for measures of epileptic activity in patients.
| Duration (years) | Chalfont—GTC | Chalfont—A | Chalfont—MCJ | |
|---|---|---|---|---|
| Mean | 20.2 | 91 | 20 | 11 |
| IQR | — | 105 | 15 | 4 |
| SD | 15.2 | — | — | — |
| Range | 2–71 | 0–191 | 0–135 | 0–122 |
| Total no—GTC | Total no—A | Total no—MCJ | |
|---|---|---|---|
| Mean | 261 | 12 165 | 11 291 |
| IQR | 159 | 998 | 200 |
| SD | — | — | — |
| Range | 0–3125 | 0–182 500 | 0–182 500 |
High scores indicate severely affected.
Data are not normally distributed, except Duration.
A, absence; Chalfont, Chalfont Seizure Severity Scale; GTC, generalised tonic–clonic seizure; IQR, interquartile range; MCJ, myoclonic jerk; Total no, total number.
Group comparisons of neuropsychology
The patients performed significantly worse than the controls on 7 of the 12 memory tests. Table 3 summarises these results.
Table 3 Summary of group comparisons for each of the memory tests.
| Test | p Value |
|---|---|
| Significant | |
| Face Recognition | 0.001 |
| D&P: People | 0.004 |
| D&P: Doors | 0.004 |
| D&P: People Delayed | 0.005 |
| D&P: Shapes Delayed | 0.035 |
| Complex Figure Recall: Immediate | 0.05 |
| Complex Figure Recall: Delayed | 0.062* |
| Non‐significant | |
| D&P: Shapes | 0.109 |
| Word Recognition | 0.346 |
| Story Recall: Immediate | 0.442 |
| Story Recall: Delayed | 0.519 |
| D&P: Names | 0.817 |
The statistical significance of the analysis is shown in the second column—the patients performed worse than controls in all significant results. The results are ordered by statistical significance, with the lowest p value at the top.
D&P, Doors and People.
*Reported in the Results section as approaching significance.
IQ and cognitive processing
Mann–Whitney U tests showed no significant difference in mean NART IQ scores between patients (mean 98 (SD 13.4)) and controls (mean 104 (SD 8.1); Z = −1.710; p = 0.087). A significant difference was, however, observed between patients (mean 93 (SD 14.2)) and controls (mean 106 (SD 15.8); Z = −2.465; p = 0.014) in WAIS‐R IQ. Wilcoxon's signed rank test showed no significant difference between NART IQ (mean 104 (SD 8.1)) and WAIS‐R IQ (mean 106 (SD = 15.8); Z = −0.483; p = 0.629) in the group of healthy controls. A significant difference, was, however, observed between the two measures in the patient group (NART IQ mean 98 (SD 13.4); WAIS‐R IQ mean 93 (SD = 14.2); Z = −2.510; p = 0.012). The difference between the two measures did not correlate with duration of epilepsy (r = 0.234; n = 28; p = 0.230) in the patient group. The estimated total numbers of all types of seizures and the Chalfont Seizure Severity Scale scores were too restricted to enable regression and correlation analysis of these measures with any other variables.
Independent samples t tests showed that the patients performed worse than controls in both the Speed of Comprehension section (patients mean 53 (SD 19.4); controls mean 67 (SD 16.4); t (40) = −2.229; p = 0.031) and the Spot the Word section (patients mean 46 (SD 6.3); controls mean 51 (SD 3.4); t (39) = −2.426; p = 0.020) of SCOLP.
Story recall
The Mann–Whitney U test showed no significant difference between patients and controls in either the immediate recall (patients mean 9.3 (SD 6.3); controls mean 9.5 (SD 4.1); Z = −0.769; p = 0.442) or delayed recall (patients mean 7.8 (SD 5.4); controls mean 8.1 (SD 3.7); Z = −0.645; p = 0.519) of the Story Recall Test. Wilcoxon signed rank test confirmed a significant difference between the immediate and the delayed recall scores on the Story Recall Test for the patients (immediate mean 9.3 (SD 6.3); delayed mean 7.8 (SD 5.4); Z = −3.594; p<0.001) and controls (immediate mean 9.5 (SD 4.1); delayed mean 8.1 (SD 3.7); Z = −2.915; p = 0.004).
Complex figure recall
All the healthy controls scored 10/10 on the copy section of the Complex Figure Recall. Ten of the patients, however, made at least one mistake. Six patients scored 9, three patients scored 8 and one patient scored 7.
Wilcoxon's signed rank test showed that both patients (immediate mean 8.3 (SD 1.9); delayed mean 7.6 (SD 2.1); Z = −2.494; p = 0.013) and controls (immediate mean 9.2 (SD 1.5); delayed mean 8.7 (SD 1.9); Z = −2.070; p = 0.038) scored significantly worse on the delayed recall section than on the immediate recall section of the Complex Figure Recall.
The Mann–Whitney U test showed a significant difference between the scores of the patients and controls on the immediate recall section (patients mean 8.3 (SD 1.9); controls mean 9.2 (SD 1.5); Z = −1.964; p = 0.050) of the Complex Figure Recall Test and that the difference between the scores of the patients and controls on the delayed recall section (patients mean 7.6 (SD 2.1); controls mean 8.7 (SD 1.9); Z = −1.864; p = 0.062) approached significance.
D&P
The Mann–Whitney U test was used to compare the scores of patients and controls on the People, Doors, Shapes and Names Tests. The patient group scored significantly worse than the control group in the People Test (patients mean 24 (SD 7.6); controls mean 31 (SD 2.5); Z = −2.902; p = 0.004), the Doors Tests (patients mean 18 (SD 3.9); controls mean 21 (SD 2.5); Z = −2.869; p = 0.004), the People Delayed Test (patients mean 9.1 (SD 2.9); controls mean 11.5 (SD 0.9); Z = −2.812; p = 0.005) and the Shapes Delayed Test (patients mean 10.8 (SD 2.0); controls mean 11.9 (SD 0.3); Z = −2.107; p = 0.035). We found no significant difference in scores between patients and healthy controls for the Shapes Test (patients mean 32 (SD 7.0); controls mean 35 (SD 2.6); Z = −1.604; p = 0.109) and the Names Test (patients mean 19 (SD 2.9); controls mean 20 (SD 1.6); Z = −0.232; p = 0.817).
The immediate and delayed scores of the patients and the healthy controls on the People Test and the Shapes Test were compared by Wilcoxon's signed rank test. This difference reflects the rate of forgetting. In the People Test, we found a significant difference between the two conditions in the patient group (immediate mean 11 (SD 2.3); delayed mean 9 (SD 2.9); Z = −3.172; p = 0.002) but not in the healthy control group (immediate mean 12 (SD 0.3); delayed mean 12 (SD 0.9); Z = −1.663; p = 0.102). In the Shapes Test, we found no significant difference between the two conditions in the patients (immediate mean 11 (SD 2.4); delayed mean 11 (SD 2.0); Z = −0.916; p = 0.360) and in the controls (immediate mean 12 (SD 0.3); delayed mean 12 (SD 0.3); Z = 0.000; p = 1.000).
Face and word recognition
The Mann–Whitney U test showed a significant difference in scores on the face recognition test between patients (mean 44 (SD 4.3)) and controls (mean 47 (SD 2.4); Z = −3.228; p = 0.001). We found no significant difference between patients and controls in the scores for the Word Recognition Test (patients mean 48 (SD 2.370); controls mean 49 (SD 1.163); Z = −0.943; p = 0.346).
Group comparisons of MRS
The independent samples t test showed no significant differences between the patients and controls on any of the MRS ratios when comparing the right, left and summary values (table 4).
Table 4 Results of independent samples t test comparing magnetic resonance spectroscopy results of patients and controls.
| Controls Mean (SD) | Patients Mean (SD) | Statistics | |
|---|---|---|---|
| Right‐hand side | |||
| NA:Cho | 1.63 (0.75) | 1.39 (0.41) | t (31) = −1.187, p = 0.244 |
| NA:Cr | 1.52 (0.42) | 1.36 (0.34) | t (31) = −1.183; p = 0.246 |
| Cho:Cr | 0.99 (0.20) | 1.03 (0.30) | t (31) = 0.360; p = 0.722 |
| Left‐hand side | |||
| NA:Cho | 1.51 (0.35) | 1.30 (0.32) | t (25) = −1.649; p = 0.112 |
| NA:Cr | 1.42 (0.25) | 1.29 (0.29) | t (25) = −1.263; p = 0.218 |
| Cho:Cr | 0.96 (0.15) | 1.00 (0.14) | t (25) = 0.830; p = 0.415 |
| Summary value | |||
| NA:Cho | 1.48 (0.47) | 1.30 (0.35) | t (34) = −1.271; p = 0.212 |
| NA:Cr | 1.43 (0.30) | 1.29 (0.32) | t (34) = −1.219; p = 0.231 |
| Cho:Cr | 0.99 (0.16) | 1.02 (0.22) | t (34) = 0.368; p = 0.715 |
Cho, choline; Cr, creatine; NA, N‐acetyl aspartate.
Relationships between MRS and neuropsychology
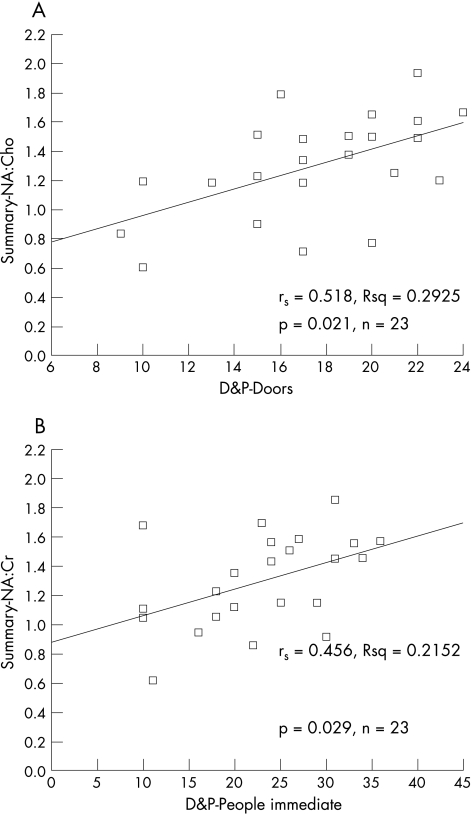
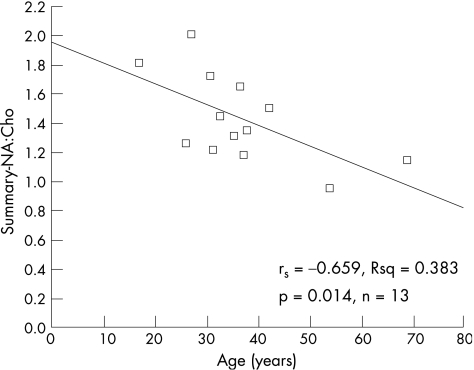
Spearman's rank‐order correlation and scatter graphs were used to investigate the relationships between MRS ratios and the results of neuropsychological testing. Data from the Complex Figure Recall were not analysed because the range of scores was restricted. Table 5 shows significant correlations from the analyses of NA:Cho and NA:Cr with neuropsychology. Figure 2 shows the scatter graphs for two of the strongest correlations between memory and MRS ratios in the patients and fig 3 shows the scatter graph for age against summary MRS in the controls.
Table 5 Significant correlations between magnetic resonance spectroscopy ratios (NA:Cho and NA:Cr in the right temporal lobe, the left temporal lobe and the summary measure), neuropsychology data and age.
| NA:Cho | NA:Cr | |
|---|---|---|
| Patient summary | ||
| D&P: Doors* | rs = 0.518; p = 0.011; n = 23 | rs = 0.571; p = 0.004; n = 23 |
| D&P: People* | rs = 0.418; p = 0.047; n = 23 | rs = 0.456; p = 0.029; n = 23 |
| Face Recognition* | rs = 0.479; p = 0.021; n = 23 | rs = 0.468; p = 0.24; n = 23 |
| Story Recall: Immediate | NS | rs = 0.549; p = 0.028; n = 23 |
| Patients right | ||
| Face Recognition* | rs = 0.660; p = 0.001; n = 21 | rs = 0.506; p = 0.019; n = 21 |
| Story Recall: Immediate | NS | rs = 0.544; p = 0.011; n = 21 |
| Story Recall: Delayed | NS | rs = 0.514; p = 0.017; n = 21 |
| Controls summary | ||
| Age | rs = −0.659; p = 0.014; n = 13 | rs = −0.593; p = 0.033; n = 13 |
Cho, choline; Cr, creatine; NA, N‐acetyl aspartate; rs, Spearman's correlation coefficient.
*Patients performed significantly worse than controls. NS, not significant.
Figure 2 Scatter graphs showing the relationship between (A) Doors and People (D&P): Doors, and summary N‐acetyl aspartate:choline (NA:Cho) and (B) D&P: People Immediate, and summary N‐acetyl aspartate:creatine (NA:Cr) in patients with idiopathic generalised epilepsy. rs, Spearman's correlation coefficient.
Figure 3 Scatter graph showing the relationship between summary N‐acetyl aspartate:choline and age in the control group. rs, Spearman's correlation coefficient.
In the control group, left NA:Cho correlated with delayed story recall (r = 0.612; n = 11; p = 0.046). Partial correlation was used to explore the relationship in the controls between left NA:Cho and delayed story recall while controlling for age. Preliminary analyses were carried out to ensure that no violation of the assumptions of normality, linearity and homoscedasticity occurred. We found a strong positive correlation between NA:Cho and delayed story recall (r = 0.60; n = 11; p = 0.049). An inspection of the zero‐order correlation, however, suggested that age had a marked effect on the strength of the correlation between these variables, which was no longer significant (r = 0.48; n = 11; p = 0.161) when the effect of age was removed.
Relationships between seizure activity and other measures
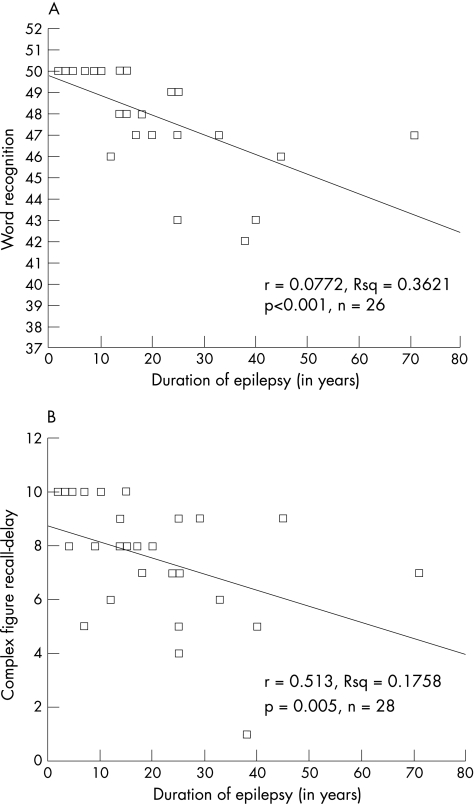
We found statistically significant negative correlations in the patients between duration of epilepsy and scores on four of the neuropsychological tests: the delayed recall section of Complex Figure Recall (r = −0.513; n = 28; p = 0.005), Word Recognition (r = −0.772; n = 26; p<0.001), the D&P: People Test (r = −0.384; n = 28; p = 0.044) and the D&P: Names Test (r = −0.475; n = 28; p = 0.011). Figure 4 shows scatter graphs for two of these correlations.
Figure 4 Scatter graphs showing the relationship between (A) word recognition scores and (B) Doors and People: People, and duration of epilepsy in the patients with idiopathic generalised epilepsy.
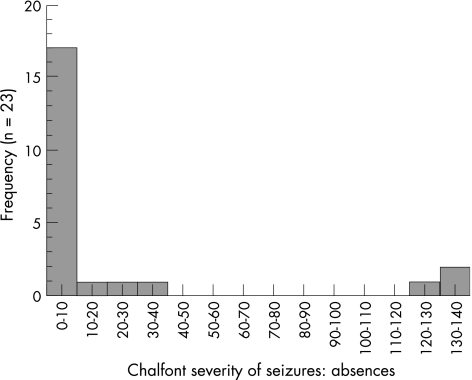
The estimated total numbers of all types of seizures and the Chalfont Seizure Severity Scale scores were too restricted to enable regression and correlation analysis. Therefore, it was not possible to establish the existence of any relationships between seizure activity and either MRS or neuropsychological test scores. Table 2 shows summary data for numbers of each type of seizure and fig 5 shows a typical histogram for seizure activity, illustrating the restricted distribution.
Figure 5 Histogram for scores on the absence section of the Chalfont Seizure Severity Scale.
Discussion
The main conclusions of the study can be summarised as follows:
Generalised cognitive deficit: Current IQ of the patients (WAIS–R) was significantly lower than their premorbid IQ (NART), which suggests a generalised cognitive decline. Consistent with this conclusion, patients also performed worse than controls on both sections of SCOLP and made numerous mistakes on the copy section of the Complex Figure Recall.
Memory impairments: Eighty six per cent of the patients and none of the healthy controls reported memory impairment. The patients performed worse than controls in 7 of the 12 memory tests. We found no apparent predilection for poor performance in any of the major memory domains; deficits were found with comparable frequency in recall, recognition, visual and verbal memory tests. Two of the four tests assessing memory after a delay showed that the patients forgot faster than the controls. In the D&P: People Test, a significant difference was seen between the patients and controls in both immediate recall and delayed recall, but within‐group comparisons showed that only the patients performed worse on the delayed recall than on the immediate recall. Patients performed significantly worse than the controls on the delayed recall section of the D&P: Shapes Test. In the Complex Figure Recall Test, the patients performed worse than the controls in both immediate and delayed recall conditions, but the rate of forgetting was not significantly different. Both groups showed similar forgetting rates in the Story Recall Test.
Correlation between memory performance and MRS: The patients' MRS ratios correlated with their scores on several memory tests. This relationship may be due to neuronal dysfunction caused by epileptic activity. Although the MRS data correlated with some of the measures of memory performance, we found no overall difference between group means for any of the MRS measures. Inspection of group means, standard deviation, range and histograms showed that the two groups were almost identical. In particular, we found that no subgroup of patients had low ratios, and neither was there any evidence of a focal or lateralised impairment of cerebral function or performance preferentially affecting specific domains of memory.
Whilst none of the patients were tested within 24 hours of a GTCS (most had not had a GTCS for much longer), it is possible that subclinical seizure activity during the testing could affect their performance on the neuropsychological tests. The relationship between cognitive impairment and abnormalities on EEG is not straightforward, and there is no simple way of excluding such an effect even with continuous EEG monitoring of participants during the test session. There was, however, no evidence of such seizure activity during the testing sessions and no apparent fluctuation in the performance during the sessions.
It is possible that the cognitive and memory dysfunction observed in the patient group was secondary to drug treatment. The influence of epilepsy drugs on cognitive performance has recently been reviewed.31,32 Valproate is generally considered to have relatively mild cognitive effects, but the evidence is inconclusive. The effects of drugs are dose dependent and polytherapy may slow cognitive performance more than monotherapy. The possibility that cognitive performance and MRS are correlated via a common effect of epilepsy drugs cannot be excluded and future larger studies should incorporate current drug treatment into their recruitment criteria to allow statistical analysis to include this variable.
It was not possible to analyse the relationship between seizure activity and memory performance because of the restricted range of distribution of the data on seizures. Although the results show clear correlations between four of the neuropsychological tests and duration of epilepsy, which may indicate a relationship between epileptic activity and memory performance, a longer duration of epilepsy does not necessarily imply a higher total lifetime number of seizures or a higher frequency of seizures. People with epilepsy of long duration may have had fewer seizures than people with recent onset and vice versa. Or it may simply be that this result reflects decreased performance on these tests with age. Future studies should adjust recruitment criteria to ensure selection of patients with a wide range of seizure activity. These data would enable more powerful conclusions to be drawn on the relationship between seizure activity, memory performance and neuronal dysfunction.
In conclusion, this study has shown that patients with IGE perform worse than age‐matched controls on a range of memory tests and that this deficit correlates with MRS measures of neuronal dysfunction in the temporal lobes. Our hypothesis is that seizure activity itself causes neuronal dysfunction in the medial temporal lobes, which results in reduced performance on tests of memory and general cognition. These results merit further investigations.
Acknowledgements
We thank the staff of the radiography department of the University of Sheffield's MR Unit at the Royal Hallamshire Hospital, whose expertise was invaluable. We thank Claire Isaac for taking time to read the paper and for her useful comments.
Abbreviations
Cho - choline
Cr - creatine
D&P Test - Doors and People Test
EEG - electroencephalogram
GTCS - generalised tonic–clonic seizure
IGE - idiopathic generalised epilepsy
MRS - magnetic resonance spectroscopy
NA - N‐acetyl aspartate
NART - National Adult Reading Test
SCOLP - speed and capacity of language processing
TLE - temporal lobe epilepsy
WAIS—R - Wechsler Adult Intelligence Scale—Revised
Footnotes
Competing interests: None.
References
- 1.Mattson R. Overview: idiopathic generalised epilepsies. Epilepsia 200344(Suppl 2)2–6. [DOI] [PubMed] [Google Scholar]
- 2.Anon Commission on Classification and Terminology of Epilepsies and Epileptic Syndromes Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 198930389–399. [DOI] [PubMed] [Google Scholar]
- 3.Giovagnoli A, Avanzini G. Quality of life and memory performance in patients with temporal lobe epilepsy. Acta Neurol Scand 2000101295–300. [DOI] [PubMed] [Google Scholar]
- 4.Mungas D, Cindy E, Walton N.et al Verbal learning differences in epileptic patients with left and right temporal lobe foci. Epilepsia 198526340–345. [DOI] [PubMed] [Google Scholar]
- 5.Helmstaeder C, Polh C, Hufnager A.et al Visual learning in non‐resected patients with right temporal lobe epilepsy. Cortex 198727547–555. [DOI] [PubMed] [Google Scholar]
- 6.Dupont S, van de Moortele P, Samson S.et al Episodic memory in left temporal lobe epilepsy: a functional MRI study. Brain 20001231722–1732. [DOI] [PubMed] [Google Scholar]
- 7.Falk M, Cole L, Glosser G. Pseudoword and real word memory in unilateral temporal lobe epilepsy. J Clin Exp Neuropsychol 200224327–334. [DOI] [PubMed] [Google Scholar]
- 8.Lespinet V, Bresson C, N'Kaoua B.et al Effect of age of onset of temporal lobe epilepsy on the severity and the nature of preoperative memory deficits. Neuropsychologia 2002401591–1600. [DOI] [PubMed] [Google Scholar]
- 9.Jefferys J. Hippocampal sclerosis and temporal lobe epilepsy: cause or consequence? Brain 19991221007–1008. [DOI] [PubMed] [Google Scholar]
- 10.Scoville W, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 19572011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behav Brain Res 1999103123–133. [DOI] [PubMed] [Google Scholar]
- 12.Dupont S, Samson Y, Van de Moortele P ‐ F.et al Bilateral hemispheric alteration of memory processes in right medial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 200273478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammen T, Stefan H, Ederhardt K.et al Clinical applications of 1H‐MR spectroscopy in the evaluation of epilepsies—what do pathological spectra stand for with regard to current results and what answers do they give to common clinical questions concerning the treatment of epilepsies? Acta Neurol Scand 2003108223–238. [DOI] [PubMed] [Google Scholar]
- 14.Connelly A, Paesschen W V, Porter D.et al Proton magnetic resonance spectroscopy in MRI‐negative temporal lobe epilepsy. Neurology 19985161–66. [DOI] [PubMed] [Google Scholar]
- 15.Gadian D. N‐acetylaspartate and epilepsy. Magn Reson Imaging 1995131193–1195. [DOI] [PubMed] [Google Scholar]
- 16.Cendes F, Caramanos Z, Andermann F.et al Proton magnatic resonance spectroscopic imaging and magnetic resonance imaging volumetry in the lateralisation of temporal lobe epilepsy: a series of 100 patients. Ann Neurol 199742737–746. [DOI] [PubMed] [Google Scholar]
- 17.Dautry C, Vaufrey F, Brouillet E.et al Early N‐acetylaspartate depletion is a marker of neuronal dysfunction in rats and primates chronically treated with the mitochondrial toxin 3‐nitropropionic acid. J Cereb Blood Flow Metab 200020789–799. [DOI] [PubMed] [Google Scholar]
- 18.Birken D, Oldendorf W. N‐acetyl‐L‐aspartic acid: a literature review of a compound prominent in 1H‐NMR spectroscopic studies of brain. Neurosci Biobehav Rev 19891323–31. [DOI] [PubMed] [Google Scholar]
- 19.Urenjak J, Williams S, Gadian D.et al Specific expression of N‐acetylaspartate in neurons, oligodendrocyte‐type‐2 a, oligodendrocyte‐type‐2 astrocyte progenitors, and immature oligodendrocytes in vitro. J Neurochem 19925955–61. [DOI] [PubMed] [Google Scholar]
- 20.Howe F, Maxwell R, Saunders S.et al Proton spectroscopy in vivo. Magn Reson Q 1993931–59. [PubMed] [Google Scholar]
- 21.Meencke H. Neuron density in the molecular layer of the frontal cortex in primary generalised epilepsy. Epilepsia 198526450. [DOI] [PubMed] [Google Scholar]
- 22.Woermann F, Free S, Koepp M.et al Abnormal cerebral structure in juvenile myoclonic epilepsy demonstrated with voxel‐based analysis of MRI. Brain 19991222101–2108. [DOI] [PubMed] [Google Scholar]
- 23.Savic I, Lekvall A, Greitz D.et al MR spectroscopy shows reduced frontal lobe concentrations of N‐acetyl aspartate in patients with juvenile myoclonic epilepsy. Epilepsia 200041290–296. [DOI] [PubMed] [Google Scholar]
- 24.Namer I, Bolo N, Nguyen V.et al Combined measurements of hippocampal N‐acetyl‐aspartate and T2 relaxation time in the evaluation of mesial temporal lobe epilepsy: correlation with clinical severity and memory performances. Epilepsia 1999401424–1432. [DOI] [PubMed] [Google Scholar]
- 25.Duncan J, Sander J. The Chalfont Seizure Severity Scale. J Neurol Neurosurg Psychiatry 199154873–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baddeley A, Emslie H, Nimmo‐Smith I.Doors and people manual. Bury St Edmonds, UK: Thames Valley Test Company, 1994
- 27.Nelson H E.The National Adult Reading Test (NART): test manual. Windsor, UK: NFER‐Nelson, 1982
- 28.Wechsler D.WAIS‐R manual. New York: The Psychological Corporation, 1981
- 29.Baddeley A, Emslie H, Smith I.The speed and capacity of language‐processing test manual. Bury St Edmunds, UK: Thames Valley Test Company, 1992
- 30.Perneger T. What's wrong with Bonferroni adjustments? BMJ 19983161236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aldenkamp A, De Krom M, Reijs R. Newer antiepileptic drugs and cognitive issues. Epilepsia 200344(Suppl 4)21–29. [DOI] [PubMed] [Google Scholar]
- 32.Motamedi G, Meador K. Antiepileptic drugs and memory. Epilepsy Behav 2004435435–439. [DOI] [PubMed] [Google Scholar]