Abstract
Background
Post‐traumatic amnesia (PTA) tests that record different PTA durations in the same patient, thereby raising measurement accuracy issues, have been reported previously. A major problem lies in determining the end point of PTA.
Aims
To delineate areas of discrepancy in PTA tests and to provide independent verification for a criterion signalling emergence from PTA.
Methods
In a randomised design, two related PTA procedures were compared, one purportedly more difficult (Westmead PTA Scale, WPTAS) than the other (Modified Oxford PTA Scale, MOPTAS). Eighty two patients in the early stages of PTA were examined daily until emergence, by using the Galveston Orientation and Amnesia Test (GOAT) and the WPTAS/MOPTAS. A short battery of cognitive and behavioural measurements was made on three occasions: at the early stage of PTA (time 1), towards the end of PTA when the maximum score (12/12) was first obtained (time 2) and at the traditional criterion for emergence (scoring 12/12 for 3 consecutive days; time 3).
Results
No significant difference was recorded in PTA duration between the MOPTAS and WPTAS. Both scales recorded longer PTA durations than the GOAT. By using Kaplan–Meier survival analyses, the WPTAS was found to show a more protracted pattern of emergence at the end stage of PTA than the MOPTAS. A time lag of ⩾1 week in the resolution of disorientation as compared with amnesia was observed in 59% cases. Significant improvements occurred on all independent measurements between time 1 and time 2, but on only 2 of 5 cognitive measurements between time 2 and time 3.
Conclusions
Although no significant differences in the duration of PTA on the MOPTAS/WPTAS were recorded, emergence from the late stages of PTA occurred more promptly with the MOPTAS. The need for inclusion of both orientation and memory items in PTA tests is highlighted by the frequency of disorientation–amnesia dissociations. The patterns of results on the independent measures suggest that patients who are in PTA for > 4 weeks have probably emerged from PTA when they first score 12/12 on the MOPTAS/WPTAS, and this criterion can replace the traditional criterion.
Post‐traumatic amnesia (PTA) is a transitory state between coma and return of full consciousness defined as “an interval during which the patient is confused, amnesic for ongoing events and likely to evidence behavioral disturbance”.1 It is a characteristic feature of traumatic brain injury, present in about 70% of admissions to brain injury rehabilitation units.2,3 Duration of PTA is extremely variable, ranging from minutes to months. Although the early stages of PTA are easily recognised, identifying the end point is difficult and complex.4,5,6 In some cases, the end of PTA cannot be determined because of chronic memory impairment;7,8 in others, everyday behaviours at the ward level may indicate resolution of PTA, although the criterion on PTA tests cannot be achieved.9 Additionally, differences in recorded PTA duration in the same patient have been shown by using different PTA tests.10,11,12,13 Such problems raise validity issues regarding the capacity of PTA tests to accurately measure PTA duration.
More specifically, in comparing two similar scales, Tate et al13 found greater difficulty in measuring emergence from the end stages of PTA with the Westmead PTA Scale (WPTAS)14,15 than with the Modified Oxford PTA Scale (MOPTAS; unpublished). The maximum score (12/12) was obtained on a significantly larger number of occasions on the WPTAS before reaching the criterion for emergence from PTA (first of three consecutive days scoring 12/12). We describe one case where the maximum score first occurred on day 14 post trauma, but the first of three consecutive maximum scores required an additional 17 days of testing (to day 31 post trauma); the patient scored 12/12 on seven separate occasions in the interim. The more demanding method of measuring the picture recognition‐memory component was mooted as responsible, but a definitive conclusion could not be drawn. An implication of the findings was that the criterion score for emergence from PTA on the WPTAS needed reconsideration. No empirical study has been conducted for either scale to establish such a criterion. On the basis of data from Tate et al,13 an earlier time point (first occasion scoring 12/12) was a possible alternative. We reasoned that if there were no improvements on independent neurobehavioural measures between the first and third consecutive days of scoring 12/12, then a revised criterion for the end of PTA (namely, first 12/12) was justified. As the case described above shows, not all patients can immediately sustain the maximum score for three consecutive days.
Results from that study also indicated that further investigation on dissociations in resolution of disorientation and amnesia was required. Discrepancies among reports13,16 may be resolved by examining the time lag between resolution of these PTA components. If the latencies are not significant, it is not important to measure both disorientation and amnesia, and PTA tests can be simplified, as is the current trend.12,17 If large dissociations exist, however, the composition of PTA tests requires inclusion of both orientation and memory items to have any claims of accuracy.
These issues formed the aims of the study. It was hypothesised that (1) recorded duration of PTA is longer on the WPTAS than on the MOPTAS, (2) sustaining the maximum score is more difficult on the WPTAS than on the MOPTAS, and (3) differences occur on the picture recognition‐memory but not on orientation items. Sustaining the maximum score was taken to be an index of the efficiency of measuring emergence from PTA (see Method section for operational definition). The second aim was to further examine the end point of PTA with independent measures and compare three time points: at the early stage of PTA (time 1), towards the end of PTA (time 2) and at the end of PTA (time 3). As examination occasions were linked to the level of recovery (measured by PTA score) rather than time post trauma, group differences were not anticipated. Marked differences were expected between time 1 and time 2, but not between time 2 and time 3.
Method
A randomised group design, guided by the revised CONSORT statement,18 was used.
Participants
Patients were recruited from three inpatient rehabilitation units. Selection criteria were as follows: age 17–65 years, recent de novo traumatic brain injury (<6 months post trauma), early stage of PTA (scoring <7/12 on MOPTAS/WPTAS), no history of neurological or psychiatric conditions, major mood disturbance or substance misuse, fluent in English and able to participate in PTA testing.
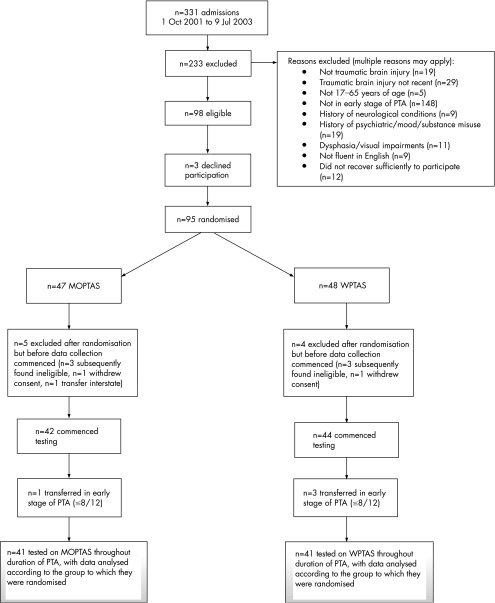
Power analysis with the data from Tate et al13 (effect size 0.48) suggested a sample size of 47 per group (power 0.80, p<0.05, one tailed). Figure 1 shows the participant flow diagram. In summary, of the 331 admissions over a 21‐month period, 95 met selection criteria and were randomised. Data collection started with 86 of 95 patients and 82 of 95 completed the study. Attrition was 13.7% (13/95). All participants remained in the group to which they were randomised, thereby meeting the basic criterion for intention‐to‐treat analyses. No significant group differences occurred on baseline measures (see table 1).
Figure 1 Participant flow diagram. MOPTAS, Modified Oxford PTA Scale; PTA, post‐traumatic amnesia; WPTAS, Westmead PTA Scale
Table 1 Descriptive data and univariate analyses for demographic and injury variables.
| Variable | MOPTAS (n = 41) Mean (SD) | WPTAS (n = 41) Mean (SD) | z† |
|---|---|---|---|
| Age at injury (years) | 29.71 (12.31) | 35.76 (15.12) | −1.80 |
| Initial GCS score (n = 69) | 5.18 (3.05) | 5.66 (2.75) | −1.11 |
| Rehabilitation admission (day post trauma) | 36.90 (26.30) | 33.27 (20.78) | −1.12 |
| PTA testing started (day post trauma) | 48.39 (29.99) | 49.32 (31.60) | −0.18 |
| Duration of PTA (GOAT; n = 81) | 77.55 (51.17) | 77.15 (53.99) | −0.29 |
| n (%) | n (%) | χ2 | |
|---|---|---|---|
| Sex | |||
| Male | 33 (80.5) | 31 (75.6) | 0.29 |
| Female | 8 (19.5) | 10 (24.4) | |
| Education (n = 79) | |||
| Student | 7 (17.5) | 3 (7.7) | 2.49 |
| High school not completed | 13 (32.5) | 14 (35.8) | |
| Completed high school | 7 (17.5) | 5 (12.8) | |
| Tertiary education | 13 (32.5) | 17 (43.6) | |
| Work status (n = 72) | |||
| Employed | 27 (79.4) | 22 (57.9) | 3.82 |
| Unemployed | 7 (20.6) | 16 (42.1) | |
| Cause of injury | |||
| Road traffic crashes | 31 (75.6) | 22 (53.7) | 5.22 |
| Fall | 6 (14.6) | 13 (31.7) | |
| Assault | 4 (9.8) | 5 (12.2) | |
| Other | 0 | 1 (2.4) |
GCS, Glasgow Coma Scale; GOAT, Galveston Orientation and Amnesia Test; MOPTAS, Modified Oxford PTA Scale; PTA, post‐traumatic amnesia; WPTAS, Westmead PTA Scale.
†Mann–Whitney U tests.
*p<0.05.
Materials
PTA measurements
A 21‐item composite PTA test was constructed, comprising items from the Galveston Orientation and Amnesia Test (GOAT),1 MOPTAS and WPTAS (see table in appendix for the listed items). Responses from the GOAT were scored in terms of weighted error points, and the total error score was subtracted from 100. The end of PTA was defined as consistent (⩾2 days) scores of >75/100. The 12‐item MOPTAS and WPTAS were both derived from procedures described by Fortuny et al.19 Each correct response was awarded one point (memory items use either free recall or recognition). PTA was deemed to have ended on the first of three consecutive days of the maximum score (12/12).
The main difference between the MOPTAS and WPTAS, which formed the independent variable, is the administration procedure for the picture recognition‐memory component. The MOPTAS uses the original procedure.19 Three target pictures are presented and recall is tested the following day. If perfect free recall is not obtained, a recognition procedure is instituted whereby target pictures are interspersed among five foils. Fortuny et al19 used 21 sets of foils, enabling presentation of new foils each day for 3 weeks, thereafter being recycled if PTA persists. The WPTAS differs in two respects: (1) foils comprise a single set of six pictures and (2) the three target pictures are changed after the first day on which the maximum score is obtained and a new set of target pictures is selected from the foils, whereas the old target pictures are then used as foils. The WPTAS method, wherein targets are used as foils, and foils as targets, makes the recognition procedure a more demanding task than the MOPTAS, in which foils and targets remain independent.
Both conditions used six foils (as per the WPTAS) to ensure equivalent administration procedures. Fifteen sets of six foils were developed for the MOPTAS by using a standardised set of pictures,20 matched to the target pictures on familiarity and visual complexity.
Duration of PTA was measured by the number of days between injury and the day post trauma on which the criterion score was obtained on the GOAT and MOPTAS/WPTAS. Orientation was measured with seven asterisked items from the composite scale (see table in appendix) used in previous studies.13,21 Disorientation was deemed to have resolved on the first of three consecutive days of perfect (free) recall. Picture recognition‐memory was measured with the three picture items, the criterion being the first of three consecutive days of perfect recognition or free recall. Statistical analyses were conducted on the following: (1) total duration (PTA, disorientation and amnesia); (2) day post trauma on which the maximum score was first obtained (12 for PTA, 7 for orientation and 3 for recognition memory); and (3) interval between the first day of the maximum score and criterion. These variables examined the ability to sustain the maximum score, which was taken as an index of the efficiency of emergence from PTA.
Independent measures
Five standard cognitive tests were administered, selection being dictated by the severe cognitive impairment of the participants at time 1: the Mini‐Mental State Examination22 as an overall screen, number of 18 targets correctly identified in the “A” Random Letter Test23 for sustained attention, milliseconds on the Simple Reaction Time subtest from the California Computerized Assessment Package24 for processing speed, the 10‐item 10‐trial version of the Verbal Selective Reminding Test (VerbalSR)25 used to validate the WPTAS14 and the Visual Selective Reminding (VisualSR) subtest from the Test of Memory and Learning.26 Alternate forms of VerbalSR were constructed, matched for word frequency.27 To minimise missing data on the learning tests, the first four trials were used (maximum correct scores were 40 for VerbalSR and 24 for VisualSR).
Two behavioural measures comprised the Agitated Behavior Scale28 (score range 14–56, with higher scores indicating greater agitation) and a brief videotaped conversation between each participant and the research staff (ATL‐B, JH or Louise Ellis) by using a standard set of questions.
Procedure
The three hospital ethics committees and the University of Sydney approved the study. Consent was obtained from the “responsible person” (usually the parent) because patients were unable to provide informed consent. Randomisation was conducted offsite and allocation was concealed by using a computer‐generated set of random numbers. These were written on inserts placed into sequentially numbered, opaque envelopes that were opened as participants were recruited to the study. Daily testing with the composite PTA scale and Agitated Behavior Scale was conducted by ATL‐B, JH and LE, who were trained and supervised by RLT and AP. Testing continued until such time as the participant emerged from PTA on the MOPTAS/WPTAS, or if this did not occur, until 6 months post trauma.
Independent measures were taken on three occasions: the early stage of PTA when the score of 7/12 was reached (time 1), towards the end of PTA when the maximum score (12/12) was first obtained (time 2) and at the end of PTA, scoring 12/12 on the third consecutive day (time 3). We recognise that at time 3 the patient has already emerged from PTA, which would occur at time 2, if indeed time 2 represents the first of three consecutive scores of 12/12. Whether time 2 represents the end of PTA by using the traditional criterion, however, can only be determined in retrospect, and issues that arise from this method are considered with supplementary analyses in the Results section. A fixed order of administration was used, with rest breaks given as required. At the conclusion of the study, the (approximately 5‐min) video sequences were edited into 16 videotapes in a pseudorandom order to ensure blinding of raters. The videotapes were independently viewed by doctors in the rehabilitation units (IJB, JEM, JAG, AEH and CK), who rated whether the patient was in or out of PTA. All raters have extensive clinical experience with patients in PTA. A group discussion/training session was conducted before the ratings.
Statistical analyses
Data collection yielded 3464 occasions of PTA testing across all participants. Occasional missing data values (because of scheduling difficulties, etc) were filled by using the average of the scores for the days preceding and following the day with missing data. Other missing data occurred because 21 of 82 (26%) participants did not meet the criterion before 6 months post trauma: 10 of 21 participants never scored 12/12. For the remaining 11 participants (4 on MOPTAS and 7 on WPTAS) who scored 12/12 at least once, imputed scores were used for some analyses, taken to be one higher than the last day of PTA testing. For example, if the last day of testing occurred at day 50 post trauma, then the score for the missing data value was taken as 51.
The three time points of independent testing yielded 224 sessions across all participants, with occasional missing data values that were left unfilled. The maximum number of missing data values on a test occurred at time 1 for VerbalSR for 5 of the 61 participants (3 on MOPTAS and 2 on WPTAS). Four participants did not have video data because families did not consent to this component and equipment failure occurred on four occasions.
Inter‐rater reliability of the videotape ratings was established by IJB and JEM on 73 segments from 48 participants, with roughly the same proportion at the three time points. The pattern of results was similar for each time point. For the combined set, inter‐rater agreement was 77%, with κ = 0.48 (p<0.001).
Data screening with Kolmogorov–Smirnov tests indicated normal distributions for most variables. Parametric and non‐parametric tests were used as appropriate. Reduced α levels (p<0.01) were used to decrease the chance of making a type 1 error owing to multiple comparisons. Kaplan–Meier survival analyses were carried out to compare patterns of emergence at the end stage of PTA.
Results
Table 2 shows data for 61 participants emerging from PTA according to the MOPTAS/WPTAS. The pattern of results was similar for the larger sample size. With one exception, all participants had emerged from PTA according to the GOAT by the time of their emergence on the MOPTAS/WPTAS. Both the MOPTAS and WPTAS recorded significantly longer PTA durations than the GOAT (t(30) = −5.28 and t(28) = −5.24, respectively, both p<0.001). When patients emerged from PTA according to the GOAT, the median MOPTAS/WPTAS score was 11/12. No statistically significant difference was recorded in PTA duration between the MOPTAS and WPTAS scores (t(59) = −0.54, p>0.05), but a larger proportion of participants tested on MOPTAS emerged immediately on obtaining the maximum score (22/36, 61%) than participants tested on WPTAS (13/36, 36%; χ2 = 4.50, df = 1, p<0.04).
Table 2 Descriptive data and univariate analyses on PTA variables for the subset of 61 participants meeting the criterion for emergence from PTA.
| Variable | MOPTAS (n = 32) Mean (SD) | WPTAS (n = 29) Mean (SD) | t |
|---|---|---|---|
| PTA as a whole | 59.29 (33.40) | 57.69 (28.06) | 0.20 |
| GOAT PTA duration (n = 60) | 68.63 (36.0) | 73.52 (34.28) | −0.54 |
| Duration of PTA on MOPTAS/WPTAS (days) | |||
| First 12/12 score (day post trauma) | 66.38 (34.04) | 64.34 (30.76) | 0.24 |
| Orientation | |||
| Duration of disorientation (days) | 62.31 (33.66) | 58.69 (27.10) | 0.46 |
| First 7/7 score (day post trauma) | 54.97 (29.13) | 50.41 (22.25) | 0.68 |
| Memory (picture recognition) | |||
| Duration of amnesia (days) | 51.00 (23.70) | 60.97 (27.12) | −1.53 |
| First 3/3 score (day post trauma) | 48.31 (23.27) | 52.72 (24.81) | −0.72 |
| Variables examining sustained maximum score | Mdn (IQR, range) | Mdn (IQR, range) | z† |
|---|---|---|---|
| PTA: days between first 12/12 score and criterion | 0 (4, 0–15) | 3 (11, 0–64) | −2.19* |
| Orientation: days between first 7/7 score and criterion | 4.0 (11, 0–36) | 2.0 (18, 0–35) | −0.36 |
| Memory: days between first 3/3 score and criterion | 0 (5, 0–21) | 4.0 (11, 0–48) | −2.59* |
GOAT, Galveston Orientation and Amnesia Test; IQR, interquartile range; Mdn, median; MOPTAS, Modified Oxford PTA Scale; PTA, post‐traumatic amnesia; WPTAS, Westmead PTA Scale.
*p<0.05.
†: Mann–Whitney U tests.
The effect size was small (0.14), explaining the non‐significant group difference. This contrasts with the medium effect size (0.48) in the study by Tate et al.13 Therefore it seems that the effect size varies as a consequence of sample characteristics. This proposition was explored by matching the present sample to the previous sample,13 by using a subset with GOAT PTA duration less than 60 days—that is, maximum GOAT PTA duration in the previous sample. Power analyses on this subset (n = 36; 18 on MOPTAS and 18 on WPTAS) showed an effect size of medium magnitude (0.49). By contrast, the effect size for the remaining 24 participants with GOAT PTA duration between 60 days and 6 months was small (0.12).
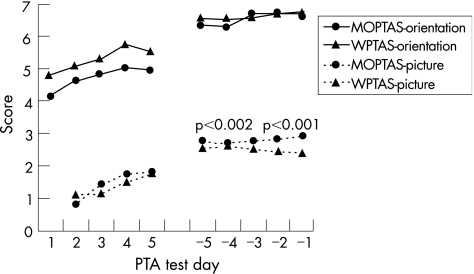
Figure 2 depicts recovery of disorientation and amnesia in the early (first 5 days) and end (last 5 days) stages of PTA, by using n = 61 meeting criterion for emergence. The orientation items showed no group differences on any data point, but significant differences for picture items occurred on the last 2 days of testing, with fewer numbers of pictures recalled in the WPTAS condition. Additionally, the interval between the first day the maximum score was obtained on the recognition‐memory items and meeting the criterion was statistically significant (z = −2.59, p = 0.01; table 2), with a longer interval occurring on the WPTAS. The effect size was of medium magnitude (0.68). No significant differences occurred for the orientation items.
Figure 2 Comparison of mean scores for the orientation and picture recognition‐memory items for the first and last 5 days of post‐traumatic amnesia (PTA) for each of the Modified Oxford PTA Scale (MOPTAS) and Westmead PTA Scale (WPTAS) conditions.
The greater number of days taken to emerge from the end stages of PTA on the WPTAS was confirmed with a Kaplan–Meier survival analysis (log rank statistic = 6.43, df = 1, p = 0.01; fig 3). In the 20 days between scoring the first 12/12 and meeting the criterion, all participants tested on MOPTAS had emerged from PTA, but about 25% from the WPTAS had not emerged. A similar result was obtained for the recognition‐memory variables (log rank statistic = 5.05, df = 1, p<0.03), but not the orientation variables (log rank statistic = 0.47, df = 1, p>0.05). All these converging findings confirm differences between the two PTA test procedures, which lie at the end stage of emergence from PTA, specifically in the picture recognition‐memory component rather than the orientation component, with emergence from PTA occurring more promptly on the MOPTAS.
Figure 3 Kaplan–Meier survival analysis on the post‐traumatic amnesia (PTA) data for the interval between the first occasion of scoring 12/12 and the third consecutive 12/12. gpoxwm, group: Oxford or Westmead condition MOPTAS, Modified Oxford PTA Scale; WPTAS, Westmead PTA Scale
Dissociations in the resolution of disorientation as compared with amnesia occurred commonly despite high correlation coefficients for the day of resolution (MOPTAS r = 0.95; WPTAS r = 0.83). Dissociations occurred in all 32 MOPTAS participants (range −12 to +41 days) and in 27 of the 29 WPTAS participants (−40 to +29 days); a negative sign indicates that orientation resolved before amnesia and a positive sign indicates that amnesia resolved before orientation. Dissociations of ⩾1 week occurred in 18 of the 32 MOPTAS (56%; orientation resolving first, n = 1; amnesia first, n = 17) and in 18 of the 29 WPTAS (61%; orientation resolving first, n = 10; amnesia first, n = 8) participants.
Examination of the independent measures on the total sample (n = 82) at the three test occasions showed no statistically significant group differences (MOPTAS v WPTAS) with respect to any of the cognitive measures, ratings of agitated behaviour or video ratings. The remaining results are therefore reported for the combined sample with testing on all three test occasions (n = 61; table 3). Significant improvement occurred on all cognitive variables, as well as the Agitated Behavior Scale, between time 1 and time 2. We found no further statistically significant improvements on three of the cognitive variables (Mini‐Mental State Examination, “A” Random Letter Test or VerbalSR) or Agitated Behavior Scale between time 2 and time 3. Although ceiling effects on the “A” Random Letter Test and possibly Mini‐Mental State Examination may have operated, no ceiling effects occurred for VerbalSR. Significant improvements occurred on the California Computerized Assessment Package and VisualSR between time 2 and time 3. For the video ratings, 42 of 56 (75%) participants were classified as being in PTA at time 1, as were 24 of 53 (45.3%) at time 2 and 16 of 51 (31.4%) at time 3.
Table 4 Independent testing for those who had emerged from post‐traumatic amnesia (PTA) at time 2 (immediate emergence) as compared with those who were still in PTA (delayed emergence) with data from the common pool (n = 61).
| Variable | Immediate emergence group | Delayed emergence group | z† |
|---|---|---|---|
| Time 1 | |||
| MMSE | 22.5 (6, 8–29) | 21.0 (7, 13–27) | −0.91 |
| ARLT | 16.5 (4, 0–18) | 16.0 (6, 3–18) | −0.17 |
| CALCAP | 644.0 (290, 315–1960) | 605.5 (342, 357–6001) | −0.18 |
| VerbalSR | 14.0 (8, 0–24) | 14.0 (6, 3–24) | −0.74 |
| VisualSR | 14.0 (8, 1–23) | 13.0 (8, 0–23) | −0.08 |
| ABS | 15.0 (3, 14–27) | 16.0 (4, 14–32) | −0.61 |
| GOAT (mean (SD)) | 62.24 (18.04) | 60.88 (13.82) | t = 0.32 |
| Time 2 | |||
| MMSE | 27.0 (4, 21–30) | 25.5 (3, 18–30) | −2.30 |
| ARLT | 18.0 (1, 14–18) | 18.0 (2, 9–18) | −1.14 |
| CALCAP | 505.0 (221, 262–1602) | 552.5 (413, 254–2431) | −1.52 |
| VerbalSR | 20.0 (7, 6–33) | 17.0 (9, 6–31) | −2.0 |
| VisualSR | 17.0 (6, 12–24) | 15.5 (7, 2–23) | −1.93 |
| ABS | 14.0 (1, 14–20) | 14.0 (3, 14–19) | −0.07 |
| GOAT (mean (SD)) | 86.83 (7.28) | 87.08 (8.18) | t = −0.13 |
| Time 3 | |||
| MMSE | 27.0 (3, 22–30) | 27.0 (4, 17–30) | −0.61 |
| ARLT | 18.0 (0, 15–18) | 18.0 (1, 14–18) | −1.52 |
| CALCAP | 442.0 (154, 251–1094) | 453.5 (158, 272–813) | −0.85 |
| VerbalSR | 20.0 (6, 7–34) | 19.0 (7, 12–30) | −0.12 |
| VisualSR | 20.0 (6, 12–24) | 20.0 (8, 7–24) | −1.03 |
| ABS | 14.0 (0, 14–20) | 14.0 (0, 14–18) | −0.03 |
| GOAT (mean (SD)) | 90.3 (6.25) | 92.54 (7.60) | t = −1.25 |
Values are median (IQR, range) unless otherwise stated.
ABS, Agitated Behavior Scale; ARLT, “A” Random Letter Test; CALCAP, California Computerized Assessment Package; GOAT, Galveston Orientation and Amnesia Test; IQR, interquartile range; Mdn, median; MMSE, Mini‐Mental Status Examination; VerbalSR, Verbal Selective Reminding Test; VisualSR, Visual Selective Reminding subtest.
†Mann–Whitney U tests.
Table 3 Independent testing for the combined sample, with testing on all three occasions (n = 61).
| Variable | Time 1 | Time 2 | Time 3 | T1 v T2 | T2 v T3 |
| z† | z† | ||||
| Cognitive tests | |||||
| MMSE | 21(6, 8–29) | 26 (4, 18–30) | 27(4, 17–30) | −6.33** | −1.38 |
| ARLT | 16 (4, 0–18) | 18 (1, 9‐18) | 18 (1, 14–18) | −5.11** | −1.59 |
| CALCAP | 622 (298, 315–6001) | 523 (294, 254–2431) | 422 (143, 251–1094) | −3.06* | −4.24** |
| VerbalSR | 14 (7, 0–24) | 19 (8, 6–33) | 20 (7, 7–34) | −5.59** | −1.44 |
| Visual SR | 14 (7, 0–23) | 16 (6, 2–24) | 20 (7, 7–24) | −4.67** | −3.97** |
| Behaviour rating | |||||
| ABS | 16 (4, 14–32) | 14 (1, 14–20) | 14 (0, 14–20) | −4.03 ** | −1.79 |
| GOAT (n = 60) Mean (SD) | 61.70 (16.06) | 86.93 (7.61) | 91.26 (6.89) | t = −12.84** | t = −4.79** |
Values are median (IQR, range) unless otherwise stated.
ABS, Agitated Behavior Scale; ARLT, “A” Random Letter Test; CALCAP, California Computerized Assessment Package; GOAT, Galveston Orientation and Amnesia Test; IQR, interquartile range; Mdn, median; MMSE, Mini‐Mental Status Examination; PTA, post‐traumatic amnesia; VerbalSR, Verbal Selective Reminding Test; VisualSR, Visual Selective Reminding subtest.
*p<0.01; **p<0.001.
†Wilcoxon signed ranks tests.
At time 2, a subset (57.4%, 35/61) had already emerged from PTA, representing those for whom time 2 (the first 12/12) was the first of three consecutive scores of 12/12. Performances of the two subgroups (“immediate emergence”, n = 35 (22 on MOPTAS and 13 on WPTAS) out of PTA at time 2 v “delayed emergence”, n = 26 (10 on MOPTAS and 16 on WPTAS) still in PTA at Time 2) were compared. Among demographic variables, statistically significant differences occurred only for age at injury (z = 2.30, p<0.03), with the delayed emergence group being older than the immediate emergence group (medians 38 v 25 years, respectively). There were, however, no significant group differences on any of the independent measures at any time point for the immediate emergence as compared with delayed emergence groups (table 4).
Table 5 shows a detailed analysis of the PTA test results for the 26 participants in the delayed emergence group, after scoring the first 12/12. It was rare for scores to drop below 10/12 in the MOPTAS condition (2%, 1/44 data points) and also uncommon in the WPTAS (11%, 18/157 data points). In all but 4 of 18 occasions, this drop in scores in the WPTAS condition occurred directly after a score of 12—that is, when the targets and foils were changed. The most commonly failed item was the picture‐memory item: at least 1 of 3 picture items were failed by 14 of 16 WPTAS participants and 5 of 10 MOPTAS participants; most WPTAS (but no MOPTAS) participants also failed multiple picture recognition‐memory items. Thereafter, the most commonly failed item was “day of the week”.
Table 5 Errors on PTA testing made between time 2 and time 3 for 26 participants (10 on MOPTAS and 16 on WPTAS) from the delayed emergence group still in PTA at time 2.
| ID | Interval | PTA scores between times 2 and 3 | Items incorrect |
|---|---|---|---|
| MOPTAS | |||
| 1 | 5 | 11, 11, 11, 11, 11 | Age×1, Month×4 |
| 2 | 6 | 10, 11, 10, 12, 12, 10 | Month×1, Day×1, EName×3, Pic1×2 |
| 3 | 4 | 11, 10, 12, 10 | Month×2, Day×2, Time×1 |
| 4 | 1 | 9 | City×1, Month×1, EName×1 |
| 5 | 4 | 11, 11, 10, 11 | Age×1, Day×3, Time×1 |
| 6 | 1 | 10 | EName×1, Pic1×1 |
| 7 | 3 | 12, 11, 11 | Day×2 |
| 8 | 9 | 11, 10, 12, 12, 11, 12, 11, 11, 11 | Month×1, Day×4, Time×1, Pic1×1 |
| 9 | 3 | 11, 11, 10 | City×1, EName×2, Pic1 ×1 |
| 10 | 8 | 10, 12, 12, 11, 11, 12, 12, 11 | Day×2, EName×1, Pic1×2 |
| WPTAS | |||
| 1 | 4 | 10, 11, 11, 11 | Day×4, Pic1×1 |
| 2 | 1 | 9 | Age×1, Hosp×1, Day×1 |
| 3 | 5 | 12, 10, 10, 12, 9 | Year×1, Pic2×3 |
| 4 | 1 | 11 | Day×1 |
| 5 | 8 | 10, 10, 10, 12, 12, 10,11, 11 | Year×1, EName×5, Pic1×2, Pic2×1 |
| 6 | 8 | 8, 10, 12, 9, 11, 12, 10, 11 | Hosp×3, EName×2, Pic1×1, Pic2×2, Pic3×1 |
| 7 | 16 | 11, 11, 12, 10, 10, 12, 10, 12, 12, 8, 12, 9, 9, 11, 12, 10 | Day×3, Pic1×2, Pic2×5, Pic3×2 |
| 8 | 15 | 11, 12, 11, 10, 11, 12, 12, 10, 12, 11, 10, 12, 9, 11, 11 | Pic1×6, Pic2×3, Pic3×1 |
| 9 | 37 | 12, 9, 10, 11, 11, 12, 10, 12, 12, 11, 11, 12, 11, 9, 11, 12, 12, 9, 10, 12, 10, 10, 12, 11, 11, 12, 11, 12, 12, 10, 12, 11, 11, 11, 12, 11, 11 | Hosp×4, Time×2, Pic1×13, Pic2×5, Pic3×2 |
| 10 | 7 | 11, 10, 10, 8, 10, 10, 11 | Year×2, Month×3, Day×4, EName×1, Pic1×4 |
| 11 | 6 | 12, 11, 10, 12, 11, 11 | Day×2, Pic1×3 |
| 12 | 2 | 8, 10 | Day×1, Pic2×1, Pic3×1 |
| 13 | 1 | 10 | Day×1, Pic1×1 |
| 14 | 3 | 10, 10, 11 | Time×1, Pic1×2, Pic2×1 |
| 15 | 11 | 9, 8, 12, 9, 10, 11, 11, 12, 10, 12, 11 | Time×2, Pic1×3, Pic2×3, Pic3×2 |
| 16 | 32 | 9, 11, 11, 10, 11, 11, 12, 10, 12, 11, 10, 12, 7, 11, 11, 11, 11, 12, 11, 10, 11, 12, 10, 11, 10, 12, 10, 10, 12, 11, 10, 11 | Month×5, Day×1, Pic1×12, Pic2×8, Pic3×2 |
EName, examiners name; MOPTAS, Modified Oxford PTA Scale; PTA, post‐traumatic amnesia; WPTAS, Westmead PTA Scale.
Interval, number of PTA test occasions between time 2 and time 3; Pic, picture memory items—data are recorded for the number of occasions that one picture only was failed (Pic 1), or, if applicable, the number of occasions that two (Pic 2) or all three (Pic 3) picture items were failed.
Discussion
Both the MOPTAS and WPTAS recorded significantly longer durations of PTA than the GOAT. Although the difference in recorded PTA duration between the MOPTAS and WPTAS was not statistically significant, sustaining the maximum score was more difficult on the WPTAS, resulting in a more protracted pattern of emergence from the end stage of PTA on that scale. Substantial evidence showed that the reason for this was the picture recognition‐memory component. The implication of the findings is that patients tested on WPTAS would have emerged from PTA much closer to the time when they first scored 12/12 (in this sample, on average at day 64 post trauma) than when the WPTAS PTA criterion was met (namely, at day 73 post trauma) if a different recognition‐memory procedure had been used, because that was the pattern that occurred for the MOPTAS (namely, days 66 and 68 post trauma, respectively).
It is clear from the inconclusive results of the video ratings that having an objective PTA scale is beneficial. Given the dissociations that were documented in the resolution of disorientation as compared with amnesia, both orientation and memory items need to be included in PTA tests. It is notable that the patterns of significant dissociations (⩾1 week) differed between conditions (amnesia resolving first in 17/18 MOPTAS, but only in 8/18 WPTAS participants), which probably reflect the difficulty level of the memory items in the WPTAS. With respect to the method of measuring memory, the type of materials described by Fortuny et al19 has advantages over historical memories (cf GOAT) in that assessment is objective, standardised and immediately verifiable. It is recognised that in the procedure described by Fortuny et al, recognition and free recall memory are given equal weighting, whereas it has been shown that recognition memory returns before free recall.13,29 Yet, the recognition procedure of the WPTAS probably taps into quite complex memory processes, such as recency memory. The WPTAS procedure is identical to that used in later studies which are unrelated to PTA. Parkin et al30 and Hunkin and Parkin 31 found clear performance differences in people with amnesia of different aetiology (Wernicke–Korsakoff syndrome v herpes simplex encephalitis). Therefore, subgroups with particular patterns of cerebral disorder may perform differentially worse on the WPTAS. In this context, the MOPTAS procedure, a test of simple encoding processes, may give a clearer picture of PTA duration in these patients.
Significant differences occurred on the independent cognitive and behavioural measures between time 1 and time 2, but few improvements occurred between time 2 and time 3. Although significant improvements occurred on tests of processing speed and visuospatial learning between time 2 and time 3, suggesting continuing recovery after the first 12/12 score, impairments in these functions are not specific to PTA and persist in many people long after emergence.32 With respect to tests of processing speed, Wilson et al7,8 found that such tests discriminated between those in PTA and groups with chronic memory impairment; yet, the singular difficulty is that of determining, within a given person, the point at which the aberrant performance becomes sufficiently normal to indicate that PTA has ended. Because of the lack of improvement on VerbalSR between time 2 and time 3, we question the traditional MOPTAS/WPTAS criterion for emergence from PTA and conclude that in samples such as the present one, emergence from PTA occurs on the first 12/12 score. Further support for this position is provided by (1) the lack of difference between the immediate and delayed emergence groups on the independent measures (including tests of processing speed and visuospatial learning) at time 2 (table 4) and (2) the individual patterns of error after scoring the first 12/12 (table 5), whereby it was uncommon for PTA scores to fall below 10/12. Moreover, the requirement for perfect scores on all items of PTA tests on consecutive occasions is contradicted by the data in Ponsford et al.33 Only 82% of their control group obtained perfect scores on the WPTAS on four consecutive occasions. The clinical relevance of reducing the criterion to the first occasion of scoring 12/12 means that PTA testing is not unnecessarily protracted in patients who hover around the maximum score—in this sample, two participants (IDs 9 and 16 in table 5) had an interval of almost 5 weeks between the first and third consecutive 12/12 score.
In aiming to determine the end point of PTA, this study raises the question, what exactly is PTA? If PTA is taken as originally described, an altered state of consciousness34 or, more recently, a confusional state,29,34 then it must have a measurable end point. Traditionally, that end point was described as “a distinct qualitative and obvious change in the patient's awareness and orientation”.35 With the introduction of standardised PTA tests, along with daily prospective assessment, emergence from PTA (at least in patients with very severe traumatic brain injury) is a gradual process.8,13 Our results further establish that emergence from PTA in this sample was characterised by improvement in different domains, at different times, with varying levels of consistency. Moreover, with very severe traumatic brain injury, the end point of PTA occurs in the context of ongoing cognitive impairments and, as a consequence, the precise transition point can be difficult to determine with current tests. One adverse consequence of the use of standardised PTA tests is that the qualitative aspects are lost and have been replaced by a quantitative and mechanistic approach, whereby PTA is defined by the test score. Our aim was to identify a criterion for emergence on PTA tests that minimised the confounding elements caused by other cognitive impairments, such as executive dysfunction and chronic memory impairments, which can artificially prolong PTA measurement.
Generalisability of the present findings is limited by the sample composition: participants were extremely severely injured and whether these findings apply to less severely injured people requires further investigation. Notwithstanding the more demanding administration procedures for the WPTAS memory items, both the MOPTAS and WPTAS provide a useful approach to measuring PTA in comparison with other scales. As screening tools, they offer many benefits in the postacute stages by providing a standardised, objective and quick assessment of the recovery process until such time as more detailed neuropsychological evaluation is indicated.
Acknowledgements
This study was supported by a grant from the Motor Accidents Authority of New South Wales, Australia. We thank Louise Ellis for helping with data collection and Melanie Leemon for her role in coordinating the early stages of the study. We also thank Professor Ian Cameron for his advice.
Abbreviations
ABS - Agitated Behavior Scale
ARLT - “A” Random Letter Test
CALCAP - California Computerized Assessment Package
GOAT - Galveston Orientation and Amnesia Test
MMSE - Mini‐Mental Status Examination
MOPTAS - Modified Oxford PTA Scale
PTA - post‐traumatic amnesia
VerbalSR - Verbal Selective Reminding Test
VisualSR - Visual Selective Reminding subtest
WPTAS - Westmead PTA Scale
APPENDIX
Composite PTA Form with items from GOAT, MOPTAS and WPTAS.
| GOAT | MOPTAS | WPTAS | |
|---|---|---|---|
| *1. Name | Y | — | — |
| 2. Address | Y | — | — |
| 3. Age | — | Y | Y |
| *4. Date of birth | Y | Y | Y |
| *5. Year | Y | Y | Y |
| *6. Month | Y | Y | Y |
| 7. Day of week | Y | Y | Y |
| *8. Day of month | Y | — | — |
| 9. Time of day | Y | — | — |
| 10. Period of day | — | Y | Y |
| *11. City | Y | Y | — |
| *12. Kind/name of place | Y | Y | Y |
| 13. Admission date | Y | — | — |
| 14. Mode of arrival | Y | — | — |
| 15. First memory after injury | Y | — | — |
| 16. Last memory before injury | Y | — | — |
| 17. Recall face | — | — | Y |
| 18. Recall name of face | — | Y | Y |
| 19–21. Recall 3 pictures | — | Y | Y |
GOAT, Galveston Orientation and Amnesia Test; MOPTAS, Modified Oxford PTA Scale; PTA, post‐traumatic amnesia; WPTAS, Westmead PTA Scale.
*Items used to examine orientation, from High et al;21 “day of the month” required the date within 5 days; “kind/name of place” required the patient to be aware that he/she was in hospital.
Footnotes
Competing interests: None.
References
- 1.Levin H S, O'Donnell V M, Grossman R G. The Galveston Orientation and Amnesia Test. A practical scale to assess cognition after head injury. J Nerv Ment Dis 1979167675–684. [DOI] [PubMed] [Google Scholar]
- 2.Tate R L, Perdices M, Pfaff A.et al Predicting duration of post‐traumatic amnesia (PTA) from early PTA measurements. J Head Trauma Rehabil 200116525–542. [DOI] [PubMed] [Google Scholar]
- 3.Nakase‐Thompson R, Sherer M, Yablon S A.et al Acute confusion following traumatic brain injury. Brain Inj 200418131–142. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed S, Bierley R, Sheikh J I.et al Post‐traumatic amnesia after closed head injury: a review of the literature and some suggestions for further research. Brain Inj 200014765–780. [DOI] [PubMed] [Google Scholar]
- 5.Glisky E, Delaney S. Implicit memory and new semantic learning in posttraumatic amnesia. J Head Trauma Rehabil 19961131–42. [Google Scholar]
- 6.McMillan T M, Jongen E L M M, Greenwood R J. Assessment of post‐traumatic amnesia after severe closed head injury: retrospective or prospective? J Neurol Neurosurg Psychiatry 199660422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson B A, Baddeley A, Shiel A.et al How does post‐traumatic amnesia differ from the amnesic syndrome and from chronic memory impairment. Neuropsychol Rehabil 19922231–243. [Google Scholar]
- 8.Wilson B A, Evans J J, Emslie H.et al Measuring recovery from post traumatic amnesia. Brain Inj 199913505–520. [DOI] [PubMed] [Google Scholar]
- 9.Tate R, Pfaff A. Problems and pitfalls in the assessment of post‐traumatic amnesia. Brain Impairment 20001116–129. [Google Scholar]
- 10.Mysiw W J, Corrigan J D, Carpenter D.et al Prospective assessment of posttraumatic amnesia: a comparison of the GOAT and the OGMS. J Head Trauma Rehabil 1990565–72. [Google Scholar]
- 11.Novack T A, Dowler R N, Bush B A.et al Validity of the Orientation Log, relative to the Galveston Orientation and Amnesia Test. J Head Trauma Rehabil 200015957–961. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz M L, Carruth F, Binns M A.et al The course of post‐traumatic amnesia – three little words. Can J Neurol Sci 199825108–116. [DOI] [PubMed] [Google Scholar]
- 13.Tate R, Pfaff A, Jurjevic L. Resolution of disorientation and amnesia during post‐traumatic amnesia. J Neurol Neurosurg Psychiatry 200068178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shores E A, Marosszeky J E, Sandanam J.et al Preliminary validation of a clinical scale for measuring the duration of post‐traumatic amnesia. Med J Aust 1986144569–572. [DOI] [PubMed] [Google Scholar]
- 15.Marosszeky N E V, Ryan L, Shores E A.et alThe PTA Protocol. Guidelines for using the Westmead Post‐Traumatic Amnesia (PTA) Scale. Sydney: Wild & Wooley, 1998
- 16.Gronwall D, Wrightson P. Duration of post‐traumatic amnesia after mild head injury. J Clin Neuropsychol 1980251–60. [Google Scholar]
- 17.Jackson W T, Novack T A, Dowler R N. Effective serial measurement of cognitive orientation in rehabilitation: the orientation log. Arch Phys Med Rehabil 199879718–720. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Schulz K F, Altman D G.et al The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 20013571191–1194. [PubMed] [Google Scholar]
- 19.Fortuny L A, Briggs M, Newcombe F.et al Measuring the duration of post traumatic amnesia. J Neurol Neurosurg Psychiatry 198043377–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snodgrass J G, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learning Memory 19806174–215. [DOI] [PubMed] [Google Scholar]
- 21.High W M, Levin H S, Gary H E. Recovery of orientation following closed‐head injury. J Clin Exp Neuropsychol 199012703–714. [DOI] [PubMed] [Google Scholar]
- 22.Folstein M, Folstein S, McHugh P. “Mini‐Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 197512189–198. [DOI] [PubMed] [Google Scholar]
- 23.Strub R L, Black F W.The mental status examination in neurology. 4th edn. Philadelphia: FA Davis, 2000
- 24.Miller E N.California Computerized Assessment Package. Los Angeles: Norland Software, 1990
- 25.Buschke H, Fuld P. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 1974241019–1025. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds C R, Bigler eds. Test of memory and learning. Austin, TX: Pro‐Ed, 1994
- 27.Thorndike E L, Lorge I.The teacher's word book of 30,000 words. New York: Teachers College, Columbia University, 1944
- 28.Corrigan J. Development of a scale for assessment of agitation following traumatic brain injury. J Clin Exp Neuropsychol 19891261–277. [DOI] [PubMed] [Google Scholar]
- 29.Stuss D T, Binns M A, Carruth F.et al The acute period of recovery from traumatic brain injury: posttraumatic amnesia or posttraumatic confusional state? J Neurosurg 199990635–643. [DOI] [PubMed] [Google Scholar]
- 30.Parkin A J, Leng N R C, Hunkin N M. Differential sensitivity to context in diencephalic and temporal lobe amnesia. Cortex 199026373–380. [DOI] [PubMed] [Google Scholar]
- 31.Hunkin N M, Parkin A J. Recency judgements in Wernicke‐Korsakoff and post‐encephalitic amnesia: influences of proactive interference and retention interval. Cortex 199329485–499. [DOI] [PubMed] [Google Scholar]
- 32.Corrigan J D, Mysiw W J, Gribble M W.et al Agitation, cognition and attention during post‐traumatic amnesia. Brain Inj 19926155–160. [DOI] [PubMed] [Google Scholar]
- 33.Ponsford J, Facem P C, Willmott C.et al Use of the Westmead PTA Scale to monitor recovery of memory after mild head injury. Brain Inj 200418603–614. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy R E, Nakase‐Thompson R, Nick T G.et al Use of the Cognitive Test for Delirium in patients with traumatic brain injury. Psychosomatics 200344283–289. [DOI] [PubMed] [Google Scholar]
- 35.Russell W R, Smith A. Post‐traumatic amnesia in closed head injury. ArchNeurol 196154–17. [DOI] [PubMed] [Google Scholar]