Abstract
The molecular basis for the profound inflammatory response and the accumulation of hyaluronan in orbital connective tissues seen in thyroid-associated ophthalmopathy is unknown. Moreover, the link between the orbital manifestations of Graves’ disease and those in the pretibial skin, localized dermopathy, has yet to be established. We have reported recently that leukoregulin, an activated T lymphocyte-derived cytokine, dramatically induces hyaluronan synthesis and prostaglandin–endoperoxide H synthase 2 in human orbital fibroblasts in culture. In the current studies, utilizing giant two-dimensional gel electrophoresis, we find that orbital fibroblasts express constitutively a protein profile that distinguishes them from skin fibroblasts derived from the abdominal wall and from the pretibium. We further demonstrate that leukoregulin, when present in culture medium for 16 hr, up-regulates a set of orbital fibroblast proteins not present in untreated cultures or in fibroblasts from the abdominal wall. However, some of the same protein inductions are present in the pretibial fibroblasts. These leukoregulin-induced changes in protein expression are completely blocked by dexamethasone (10 nM). Our findings are the first to identify proteins that appear to be expressed and differentially regulated in an anatomical site-restricted manner in orbital and pretibial fibroblasts and seem to establish a molecular link between fibroblasts from the orbit and those in pretibial skin.
Fibroblasts are a class of poorly characterized cells sharing superficial similarities with regard to their morphologies and biosynthetic activities. A number of established rodent fibroblast lines are capable of differentiating into adipocytes (1, 2) and myocytes (3). Human fibroblasts in primary culture derived from particular anatomical regions distinguish themselves by virtue of variations in cell proliferation (4), biotransformation activity (5), and responses to small regulatory molecules such as hormones, drugs, and cytokines (6–8). Moreover, discreet subsets of fibroblasts have been demonstrated within tissues (9, 10). This phenotypic diversity may represent the basis for connective tissue specialization and for the anatomical region-restricted manifestations associated with systemic diseases.
One population of human fibroblasts receiving considerable attention is that investing the orbit. Orbital fibroblasts are heterogeneous (11) and elaborate a characteristic profile of extracellular matrix components, gangliosides, and immunologic markers (12–17). They appear particularly susceptible to certain proinflammatory signals (18–20). Orbital fibroblasts play a putative role in the pathogenesis of thyroid-associated ophthalmopathy (TAO), the orbital manifestation of Graves’ disease (21). The hallmarks of TAO are (i) a dramatic increase in the accumulation of the nonsulfated glycosaminoglycan hyaluronan in orbital connective tissue and extraocular muscles and (ii) an often intense inflammatory reaction. A subgroup of patients with TAO also develop regional dermopathy, usually localized to the skin on the shin (22). Dermopathy also involves an accumulation of hyaluronan (21) and therefore shares an important characteristic with the disease process in the orbit. The proximate molecular trigger(s) involved in the activation of orbital connective tissue in TAO and skin fibroblasts of the leg in dermopathy has yet to be identified. Moreover, the molecular basis for any phenotypic similarity between fibroblasts from the orbit and shin that sets them apart from other fibroblasts has remained obscure.
We have recently reported that orbital fibroblasts appear to be especially sensitive to the lymphokine leukoregulin, a 50-kDa product of activated T lymphocytes. Leukoregulin can markedly up-regulate the synthesis of hyaluronan (23) and the expression of prostaglandin-endoperoxide H synthase 2 (PGHS-2, EC 1.14.99.1) (24), the inflammatory cyclooxygenase (25). In both instances, the cytokine actions in orbital fibroblasts were of much greater magnitude than those observed in dermal fibroblasts, making leukoregulin a candidate molecular trigger for both the pathological accumulation of hyaluronan and inflammation associated with TAO. In the current report, we have utilized giant two-dimensional protein gel electrophoresis to examine the ability of leukoregulin to influence the expression of approximately 2000 fibroblast proteins. We compare the responses of orbital fibroblast proteins with those in fibroblasts taken from pretibial and abdominal wall skin. With the exception of a few proteins, the phenotypes and the magnitude of the inductions that occur in response to leukoregulin are remarkably similar in all fibroblast strains, regardless of anatomical site of origin. However, there are two region-specific differences in the magnitude and direction of the leukoregulin responses that may have relevance to the pathogenesis of Graves’ disease and link orbital and pretibial fibroblasts. These findings document dramatic tissue-specific differences in the abilities of fibroblasts from distinct anatomical regions of the human body to alter protein expression in response to an inflammatory cytokine. They further suggest a role for such differences in the targeting of specific tissues for disease.
EXPERIMENTAL PROCEDURES
Materials.
Leukoregulin was prepared as published previously from human peripheral blood leukocytes (26). SC 58125 was kindly provided by Dr. P. Isakson (Searle). RU38486 was provided by Roussel-UCLAF. Dexamethasone was purchased from Sigma.
Cell Culture.
Orbital fibroblast strains were initiated from tissue explants of surgical waste at the time of transantral decompressive surgery for severe TAO. Dermal fibroblast strains were initiated from punch biopsies of normal-appearing tissue from the abdominal wall and the skin of the lower anterior leg. These activities have been approved by the Institutional Review Board of the Albany Medical College. A total of eight orbital fibroblast strains were studied: five from individuals with severe TAO and three from individuals without known thyroid disease. In addition, four strains of dermal fibroblasts were examined. Fibroblast cultures were allowed to proliferate to confluence as described previously (27) in 35-mm diameter plastic dishes covered with Eagle’s medium supplemented with 10% fetal bovine serum (GIBCO/BRL), antibiotics, and glutamine in a 37°C humidified incubator. Medium was changed every 3–4 days. Confluent cultures were treated with the test compounds for the periods indicated. The possibility that these cultures were contaminated with endothelial cells or smooth muscle cells was excluded by demonstrating the absence of factor VIII and smooth muscle-specific α-actin (11).
Metabolic Labeling and Two-Dimensional Protein Gel Electrophoresis.
Cell monolayers were washed and incubated with fresh methionine-free RPMI 1640 medium containing [35S]methionine (100 μCi/ml, specific activity 1200 Ci/mmol; New England Nuclear) for 60 to 90 min. Labeling was terminated by rinsing the cultures with ice-cold medium. Cell monolayers were solubilized in a buffer containing 9.5 M urea, 2% (wt/vol) Nonidet P-40, 2% (wt/vol) ampholines, and 5% (vol/vol) 2-mercaptoethanol. An aliquot was precipitated in 10% trichloroacetic acid at 4°C and subjected to liquid scintillation spectrometry.
Cellular proteins were subjected to giant format two-dimensional gel electrophoresis as described (28, 29). Briefly, proteins were separated first by isoelectric focusing in 3.3 mm × 30-cm tube gels containing 1.6% (wt/vol) ampholytes (pH 5–8; Pharmacia LKB Biotechnology) and 0.4% ampholytes (pH 3.5–10). These first dimensional gels were then annealed to the tops of 32 × 36 × 0.075 cm 10–16% exponential polyacrylamide gradient slab gels and the proteins electrophoresed. Gels were then fixed, dried, and subjected to autoradiography for 6 × 107 cpm loaded × days exposed. The resulting autoradiograms were inspected visually and, where appropriate, the peak densities of manually selected spots were quantified with a microcomputer-based densitometer (30). Densities were normalized for differences in the sensitivity of detection by scanning several spots on each gel whose intensities were invariant with respect to treatment. Quantitative differences are expressed as fold changes from control values.
RESULTS AND DISCUSSION
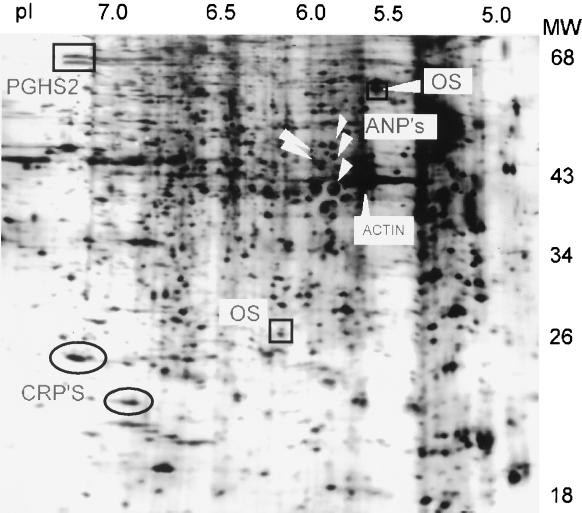
Human fibroblasts, derived from orbital connective tissue and from two distant dermal areas of a single donor with severe TAO express ca. 2000 discrete proteins resolvable by giant two-dimensional gel electrophoresis. When the proteins expressed in fibroblasts from these three origins are compared, the vast majority are similar. However, there are a few anatomical region-specific differences in basal expression of individual proteins which identify the origins of the cell strain. For example, there are two proteins which appear to be orbit-specific. OS1 (Mr 25,000, pI 6.1) and OS2 (Mr 62,000, pI 5.6) are demarcated by the boxes in Fig. 1. This figure contains a typical autoradiogram, in this case, the proteins expressed by orbital fibroblasts treated with leukoregulin. The expression of OS1 and OS2 is invariant with respect to treatment with several cytokines and glucocorticoid. Moreover, they are expressed in orbital fibroblast cultures from patients with TAO and from those without thyroid disease but not in dermal fibroblasts. Although the biological functions of OS1 and OS2 are unknown, their expression only in orbital fibroblasts underscores the consistency of phenotypic divergence in these cells.
Figure 1.
[35S]Methionine-labeled proteins expressed in human orbital fibroblasts and separated by giant two-dimensional gel electrophoresis. Shown is a typical autoradiogram of human orbital fibroblast proteins. Confluent fibroblasts from a strain derived from a patient with TAO were incubated in the presence of leukoregulin (1 unit/ml) for 15 h and metabolically labeled for 1 h. Cultures were terminated by addition of a 9.5 M urea lysis buffer and proteins separate electrophoretically as described. The symbols locate the proteins of interest: the squares locate the two orbital-specific (OS) proteins, the rectangle the 72/74 cyclooxygenase 2 protein (PGHS-2) isoforms, the ovals the CRPs, and the arrows the five ANPs.
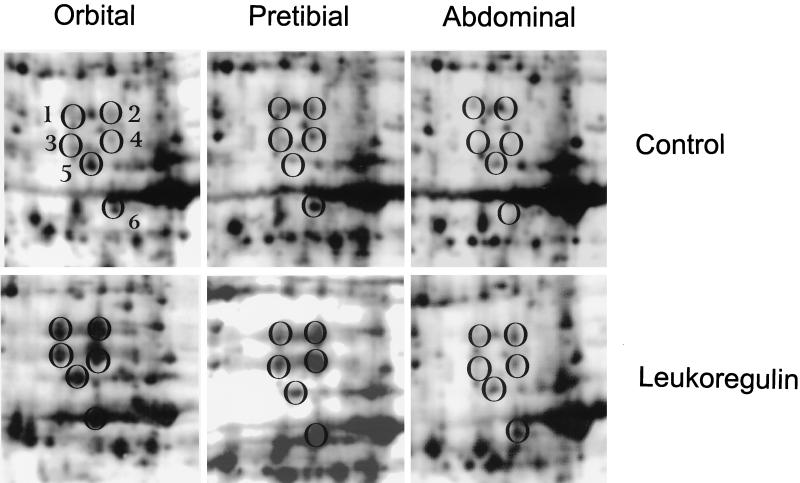
Treatment of fibroblasts in primary culture with leukoregulin (1 unit/ml for 16 h) results in marked changes in the intensities of several abundant proteins. Notable among these are two proteins, termed “cytokine-responsive proteins (CRPs),” CRP1 (Mr 24,000, pI 7.3) and CRP2 (Mr 20,000, pI 6.9), demarcated by ovals in Fig. 1. They had been recognized previously by us in other cell types and represent large inductive responses to serum and growth promoters. In these experiments, involving orbital and dermal fibroblasts, the magnitude of CRP1 and CRP2 responses to serum, interleukin 1β, and leukoregulin are approximately equivalent and they are similar in fibroblast strains taken from the three anatomical regions (Fig. 2).
Figure 2.
CRPs expressed in primary fibroblasts from orbit, abdominal wall, and pretibial skin. Confluent cultures derived from a single donor with Graves’ disease were incubated in the absence or presence of leukoregulin as described in the legend to Fig. 1. Portions of typical autoradiograms locating the CRPs are shown.
Of particular interest are the several prominent cytokine-inducible proteins exhibiting anatomical regional differences in expression. Among them is leukoregulin-induced PGHS-2 (rectangle in Fig. 1). This protein, previously identified as a 72/74 kDa doublet (30–32), is expressed at extremely low levels under basal culture conditions. In response to leukoregulin, it is induced to high levels in the orbital fibroblasts (usually much greater than 10-fold). Yet PGHS-2 is only modestly induced in dermal fibroblasts from the abdominal wall (<3-fold), and leukoregulin down-regulates PGHS-2 expression below the limits of detection in pretibial fibroblasts. We have demonstrated that steady-state PGHS-2 mRNA levels, PGHS-2 protein and prostaglandin E2 (PGE2) production are dramatically induced by leukoregulin in orbital fibroblasts (24). Addition of exogenous PGE2 or the cyclooxygenase inhibitors indomethacin (10 μM) and SC 58125 (5 μM) failed to alter the expression of any of the abundantly expressed proteins in these studies (data not shown).
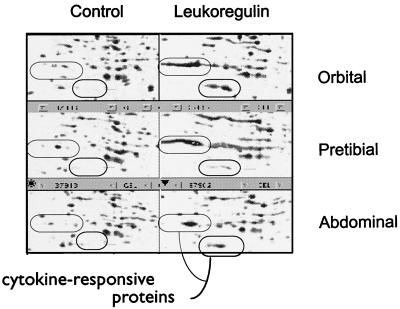
Another dramatic tissue-specific induction involves at least five proteins migrating in close proximity to actin, the “actin neighbor proteins” (ANPs; Mrs 41,000–50,000, pIs 5.8–5.9) (Figs. 1 and 3). The ANPs are expressed constitutively at very low levels in the untreated cultures of orbital and pretibial fibroblasts. However, leukoregulin treatment results in their massive up-regulation in both orbital and pretibial cultures. In contrast, these proteins do not appear to be expressed or are expressed at extremely low levels under basal or leukoregulin-stimulated conditions in abdominal wall fibroblasts. Thus, interest in these particular inductions derives from their representing responses that fulfill the expectations a priori of those that potentially link the pathological changes in orbital and pretibial connective tissue in Graves’ disease. Representative inductions of the ANPs are shown in Fig. 3. We have not observed ANP expression in other types of human or rodent cells (data not shown).
Figure 3.
ANPs in fibroblasts from three anatomical regions. Portions of typical autoradiograms locating the ANPs are shown. These autoradiograms are generated from three fibroblast strains derived from a single patient with severe TAO. Confluent cultures were treated with nothing or with leukoregulin (1 unit/ml) for 15 hr and were processed as described in Experimental Procedures. This figure demonstrates the inductions of five ANPs in orbital and pretibial dermal fibroblasts and a sixth spot that is induced massively by leukoregulin in orbital and pretibial fibroblasts and only slightly in fibroblasts from the abdominal wall.
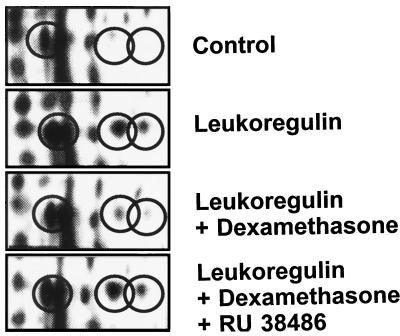
We have demonstrated previously that dramatic inductions of PGHS-2 represent characteristic responses to several cytokines, growth factors, and transforming oncogenes (24, 30–34) which act at pretranslational levels (31, 32) and that glucocorticoids substantially block the inductions of PGHS-2 mRNA, protein, and its prostaglandin synthetic activity (24, 31–35). In the case of human fibroblasts, we find that the effects of leukoregulin on PGHS-2 and ANP expression are attenuated by dexamethasone (10 nM; Fig. 4). The concentration of glucocorticoid used in these studies should saturate >90% of the cytosolic receptors (36). We find furthermore that the anti-glucocorticoid RU38486 (100 nM) substantially blocks the action of dexamethasone, restoring the inductions (Fig. 4). The observation that glucocorticoids reverse the actions of leukoregulin on ANP and PGHS-2 expression has substantial clinical relevance emanating from the widely observed benefits associated with high-dose glucocorticoid therapy in active TAO (37) and Graves’ dermopathy (38). Although the inductions of the ANPs and PGHS-2 are blocked by dexamethasone, those of the three CRPs are not, indicating that the glucocorticoid is acting through a part of the leukoregulin signaling pathway not involved in the up-regulation of the CRPs.
Figure 4.
Glucocorticoid inhibition of the leukoregulin-induced expression of the ANPs. Confluent cultures of orbital fibroblasts were incubated in the presence or absence of leukoregulin (1 unit/ml) for 15 hr. Some cultures also received dexamethasone (10 nM) without or with RU38486 (100 nM). Encircled are the three prominent acidic ANPs demonstrated in Fig. 1.
A careful examination of the behavior of the other approximately 2000 proteins synthesized by human fibroblasts reveals a few less abundant proteins that exhibit some response to leukoregulin and glucocorticoids. However, the ANPs appear to represent the only proteins we can identify the consistent behavior of which potentially links manifestations of Graves’ disease in orbital and pretibial tissues.
We describe a set of cytokine-dependent protein inductions in orbital and pretibial dermal fibroblasts that set apart these two populations of fibroblasts from that of a dermal region ordinarily not involved in Graves’ disease. Induction of the ANPs by leukoregulin occurs in orbital and pretibial fibroblast cultures but is absent in abdominal wall fibroblasts. Thus, the two extrathyroidal regions most frequently manifesting the immune consequences of Graves’ disease can, for the first time, be linked by virtue of a set of fibroblast phenotypic attributes absent in irrelevant fibroblasts. We hypothesize that the basis for anatomical-restricted inflammatory manifestations of this systemic disease in part relates to fibroblast subpopulations having differential susceptibilities to certain actions of cytokines. With regard to TAO, T lymphocytes are trafficked to the orbit (39–41). Although controversy currently exists concerning the identity of the predominant lymphocyte phenotypes found in affected orbital tissues, there exists little doubt that multiple cytokines are present, at least during the active phases of the disease. Thus our finding that orbital and pretibial fibroblasts respond to a proinflammatory lymphokine in a manner that sets them apart from other fibroblasts may represent the basis for anatomical site-restricted manifestations of the disease.
We have recently reported that leukoregulin can elicit in orbital fibroblasts a dramatic increase (up to 15-fold) in the rate of hyaluronan synthesis (23). In contrast, hyaluronan production in dermal fibroblasts is altered, but to a considerably lesser degree. This cytokine action was also attenuated by glucocorticoids (23). Expression of plasminogen activator inhibitor type 1 mRNA and protein is up-regulated by leukoregulin in orbital fibroblasts to levels that are 75-fold above baseline (16). In contrast, plasminogen activator inhibitor type 1 synthesis in dermal fibroblasts from the abdominal wall is down-regulated. Taken with the results we report here, it would appear that orbital fibroblasts exhibit a unique susceptibility to multiple actions of leukoregulin.
Comparisons of the profiles of gene expression in fibroblasts from different anatomical sites of the same donor offer the advantage of eliminating gene polymorphisms as contributing to apparent differences among strains. These studies do however raise questions about general applicability of the findings to the fibroblasts of other individuals. Moreover, the differences found in the orbital and pretibial fibroblasts demonstrated here could have been a consequence of Graves’ disease rather than inherent attributes peculiar to these fibroblasts. Thus, additional studies were conducted utilizing fibroblast strains from four other patients with TAO and from three patients without thyroid disease or orbital inflammation. Fibroblasts taken from the three normal donors exhibited tissue-specific differences in responsiveness to leukoregulin similar to those in fibroblasts from the patients with TAO.
The demonstration of tissue-specific differences in protein inducibility and susceptibility to attenuation by glucocorticoids poses some interesting questions about leukoregulin signaling mechanisms. The ability of glucocorticoids to attenuate some but not all of the cytokine’s actions on gene expression and the previously recognized activity of glucocorticoids in blocking cytokine-induced prostaglandin synthesis in many tissues (42) suggest that some inductions, particularly those of the ANPs, may be linked to prostaglandin production. Yet experiments utilizing orbital fibroblasts and employing indomethacin and SC 58125, a highly selective PGHS-2 inhibitor, failed to demonstrate alterations in the leukoregulin-dependent protein inductions. Furthermore, addition of exogenous PGE2 to cultures for 2 to 16 hr failed to produce any of these inductions. In the latter case, one cannot exclude a possible requirement for nuclear prostaglandins in the regulation of specific gene expression (43–45). This would not be fulfilled by exogenous PGE2. When taken together, however, these experiments would appear to rule against prostaglandin generation as a signal pathway utilized by leukoregulin in the induction of the noncyclooxygenase proteins. This conclusion is supported by the presence of leukoregulin-dependent ANP and CRP inductions in pretibial cells where PGHS-2 is not induced. The similarities in inductions of ANPs and CRPs in orbital and pretibial cells accompanied by divergent influences of leukoregulin on PGHS-2 in the two cell types implies the use by leukoregulin of multiple signaling pathways. This picture is reinforced by the demonstration of gene-specific attenuation of cytokine inductions by glucocorticoids rather than an overall repression of leukoregulin signaling. An activation by leukoregulin of multiple transcription factors, only some of which may be blocked by dexamethasone, such as NF-κB (46, 47) and AP1 (48), could explain all of our results, including the failure of glucocorticoids to block the induction of the CRPs which are presumably mediated by other transactivating factors. If this is indeed the case, it is likely that the fibroblast-specific differences in protein inducibility are a consequence, at least in part, of variation in the array of transcriptional factors expressed by each cell type.
There are other unresolved issues. It remains unclear whether all of the protein inductions shown here reflect increases in net synthesis or represent changes in processing. The former seems most likely with regard to PGHS-2 since in several other cell types we have found that the characteristic 72/74-kDa protein doublet on gels represents glycosylation variants of the primary translation product. Changes in abundance of this protein reflect proportional up-regulation in translatable PGHS-2 mRNA levels (31, 32, 49). Our experience with inductions of other protein clusters on bidimensional gel analysis suggests that the ANPs are likely to be closely related, possibly modifications of a single species with some representing processing products (49). Certain data support differential protein processing. In one experiment, a 6-hr leukoregulin exposure resulted in the more basic pair of ANPs being nearly as abundant as the more acidic pair (data not shown). After longer treatments (16–24 h), the inductions of the more acidic pair predominate. Verification of such relationships will await peptide mapping and in vitro translation of relevant mRNAs and/or the generation of specific probes by sequencing or molecular cloning.
Acknowledgments
We thank Matt Bowersox for expert technical assistance. This work was supported in part by National Institutes of Health Grants DK 16177, CA 56833, EY 08976, and EY 11708 and a Merit Review Award from the Department of Veterans Affairs Research Service.
ABBREVIATIONS
- ANP
actin neighbor protein
- CRP
cytokine-responsive protein
- PGE2
prostaglandin E2
- PGHS-2
prostaglandin endoperoxide H synthase 2
- TAO
thyroid-associated ophthalmopathy
References
- 1.Green H, Kehinde O. Cell. 1976;7:105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- 2.Sadowski H B, Wheeler T T, Young D A. J Biol Chem. 1992;266:4722–4731. [PubMed] [Google Scholar]
- 3.Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell T K, Turner D, Rupp R, Hollenberg S, Zhuang Y, Lassar A. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- 4.Harper R A, Grove G. Science. 1979;204:526–527. doi: 10.1126/science.432659. [DOI] [PubMed] [Google Scholar]
- 5.Pinsky L, Finkelberg R, Straisfeld C, Zilahi B, Kaufman M, Hall G. Biochem Biophys Res Commun. 1972;46:364–369. doi: 10.1016/s0006-291x(72)80147-5. [DOI] [PubMed] [Google Scholar]
- 6.Hassell T M, Page R C, Narayanan A S, Cooper C G. Proc Natl Acad Sci USA. 1976;73:2909–2912. doi: 10.1073/pnas.73.8.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith T J, Bahn R S, Gorman C A. J Clin Endocrinol Metab. 1989;69:1019–1023. doi: 10.1210/jcem-69-5-1019. [DOI] [PubMed] [Google Scholar]
- 8.Smith T J, Bahn R S, Gorman C A, Cheavens M. J Clin Endocrinol Metab. 1991;72:1169–1171. doi: 10.1210/jcem-72-5-1169. [DOI] [PubMed] [Google Scholar]
- 9.Korn J H, Torres D, Downie E. Arthritis Rheum. 1984;27:174–179. doi: 10.1002/art.1780270208. [DOI] [PubMed] [Google Scholar]
- 10.Phipps R P, Penney D P, Keng P, Quill H, Paxhia A, Derdak S, Felch M E. Am J Respir Cell Mol Biol. 1989;1:65–74. doi: 10.1165/ajrcmb/1.1.65. [DOI] [PubMed] [Google Scholar]
- 11.Smith T J, Sempowski G D, Wang H-S, Del Vecchio P J, Lippe S D, Phipps R P. J Clin Endocrinol Metab. 1995;80:2620–2625. doi: 10.1210/jcem.80.9.7673404. [DOI] [PubMed] [Google Scholar]
- 12.Smith T J, Kottke R J, Lum H, Andersen T T. Am J Physiol. 1993;265:C138–C142. doi: 10.1152/ajpcell.1993.265.1.C138. [DOI] [PubMed] [Google Scholar]
- 13.Smith T J, Ahmed A, Hogg M G, Higgins P J. Am J Physiol. 1992;263:C24–C29. doi: 10.1152/ajpcell.1992.263.1.C24. [DOI] [PubMed] [Google Scholar]
- 14.Smith T J, Higgins P J. J Invest Dermatol. 1993;100:288–292. doi: 10.1111/1523-1747.ep12469828. [DOI] [PubMed] [Google Scholar]
- 15.Smith T J, Higgins P J. Biochim Biophys Acta. 1993;1181:300–306. doi: 10.1016/0925-4439(93)90036-z. [DOI] [PubMed] [Google Scholar]
- 16.Hogg M G, Evans C H, Smith T J. Am J Physiol. 1995;269:C359–C366. doi: 10.1152/ajpcell.1995.269.2.C359. [DOI] [PubMed] [Google Scholar]
- 17.Berenson C S, Smith T J. J Clin Endocrinol Metab. 1995;80:2668–2674. doi: 10.1210/jcem.80.9.7673410. [DOI] [PubMed] [Google Scholar]
- 18.Higgins P J, Smith T J. Biochim Biophys Acta. 1993;1181:23–30. doi: 10.1016/0925-4439(93)90085-f. [DOI] [PubMed] [Google Scholar]
- 19.Smith T J, Wang H-S, Hogg M G, Henrikson R C, Keese C R, Giaever I. Proc Natl Acad Sci USA. 1994;91:5094–5098. doi: 10.1073/pnas.91.11.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H-S, Keese C R, Giaever I, Smith T J. J Clin Endocrinol Metab. 1995;80:3553–3560. doi: 10.1210/jcem.80.12.8530598. [DOI] [PubMed] [Google Scholar]
- 21.Smith T J, Bahn R S, Gorman C A. Endocr Rev. 1989;10:366–391. doi: 10.1210/edrv-10-3-366. [DOI] [PubMed] [Google Scholar]
- 22.Trotter W R, Eden K C. Q J Med. 1942;11:229–240. [Google Scholar]
- 23.Smith T J, Wang H-S, Evans C H. Am J Physiol. 1995;268:C382–C388. doi: 10.1152/ajpcell.1995.268.2.C382. [DOI] [PubMed] [Google Scholar]
- 24.Wang H-S, Cao H J, Winn V D, Rezanka L J, Frobert Y, Evans C H, Sciaky D, Young D A, Smith T J. J Biol Chem. 1996;271:22718–22728. [PubMed] [Google Scholar]
- 25.Herschman H R. Cancer Metastasis Rev. 1994;13:241–256. doi: 10.1007/BF00666095. [DOI] [PubMed] [Google Scholar]
- 26.Evans C H, Wilson A C, Gelléri B A. Anal Biochem. 1989;177:358–363. doi: 10.1016/0003-2697(89)90066-3. [DOI] [PubMed] [Google Scholar]
- 27.Smith T J. J Clin Invest. 1984;74:2157–2163. doi: 10.1172/JCI111642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young D A, Voris B P, Maytin E V, Colbert R A. Methods Enzymol. 1983;91:190–214. doi: 10.1016/s0076-6879(83)91017-0. [DOI] [PubMed] [Google Scholar]
- 29.Voris B P, Young D A. Anal Biochem. 1980;104:478–484. doi: 10.1016/0003-2697(80)90103-7. [DOI] [PubMed] [Google Scholar]
- 30.Levenson R M, Maytin E V, Young D A. Anal Biochem. 1986;158:294–301. doi: 10.1016/0003-2697(86)90553-1. [DOI] [PubMed] [Google Scholar]
- 31.O’Banion M K, Winn V D, Young D A. Proc Natl Acad Sci USA. 1992;89:4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Banion M K, Sadowski H B, Winn V, Young D A. J Biol Chem. 1991;266:23261–23267. [PubMed] [Google Scholar]
- 33.Han J-w, Sadowski H, Young D A, Macara I G. Proc Natl Acad Sci USA. 1990;87:3373–3377. doi: 10.1073/pnas.87.9.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winn V D, O’Banion M K, Young D A. J Lipid Mediators. 1993;6:101–111. [PubMed] [Google Scholar]
- 35.Levenson R, Iwata K, Klagsbrun M, Young D A. J Biol Chem. 1985;260:8056–8063. [PubMed] [Google Scholar]
- 36.Bird C C, Robertson A M G, Read J, Currie A R. J Pathol. 1977;123:145–156. doi: 10.1002/path.1711230304. [DOI] [PubMed] [Google Scholar]
- 37.Prummel M F, Mourits M P, Berghout A, Krenning E P, van der Gaag R, Koornneef L, Wiersinga W M. N Engl J Med. 1989;321:1353–1359. doi: 10.1056/NEJM198911163212002. [DOI] [PubMed] [Google Scholar]
- 38.Kriss J P, Pleshakov V, Rosenblum A, Sharp G. J Clin Endocrinol Metab. 1967;27:595–604. doi: 10.1210/jcem-27-5-595. [DOI] [PubMed] [Google Scholar]
- 39.De Carli M, D’Elios M M, Mariotti S, Marcocci C, Pinchera A, Ricci M, Romagnani S, Del Prete G. J Clin Endocrinol Metab. 1993;77:1120–1124. doi: 10.1210/jcem.77.5.8077301. [DOI] [PubMed] [Google Scholar]
- 40.Grubeck-Loebenstein B, Trieb K, Sztankay A, Holter W, Anderi H, Wick G. J Clin Invest. 1994;93:2738–2743. doi: 10.1172/JCI117289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weetman A P, Cohen S, Gatter K C, Fells P, Shine B. Clin Exp Immunol. 1989;75:222–227. [PMC free article] [PubMed] [Google Scholar]
- 42.Masferrer J L, Seibert K, Zweifel B, Needleman P. Proc Natl Acad Sci USA. 1992;89:3917–3921. doi: 10.1073/pnas.89.9.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morita I, Schindler M, Regier M K, Otto J C, Hori T, DeWitt D L, Smith W L. J Biol Chem. 1995;270:10902–10908. doi: 10.1074/jbc.270.18.10902. [DOI] [PubMed] [Google Scholar]
- 44.Kliewer S A, Lenhard J M, Willson T M, Patel I, Morris D C, Lehmann J M. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 45.Forman B M, Tontonoz P, Chen J, Brun R P, Spiegelman B M, Evans R M. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 46.Scheinman R I, Gualberto A, Jewell C M, Cidlowski J A, Baldwin A S., Jr Mol Cell Biol. 1995;15:943–953. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohtsuka T, Kubota A, Hirano T, Watanabe K, Yoshida H, Tsurufuji M, Iizuka Y, Konishi K, Tsurufuji S. J Biol Chem. 1996;271:1651–1659. doi: 10.1074/jbc.271.3.1651. [DOI] [PubMed] [Google Scholar]
- 48.Paliogianni F, Raptis A, Ahuja S S, Najjar S M, Boumpas D T. J Clin Invest. 1993;91:1481–1489. doi: 10.1172/JCI116353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Banion M K, Young D A. J Virol. 1991;65:3481–3488. doi: 10.1128/jvi.65.7.3481-3488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]