Abstract
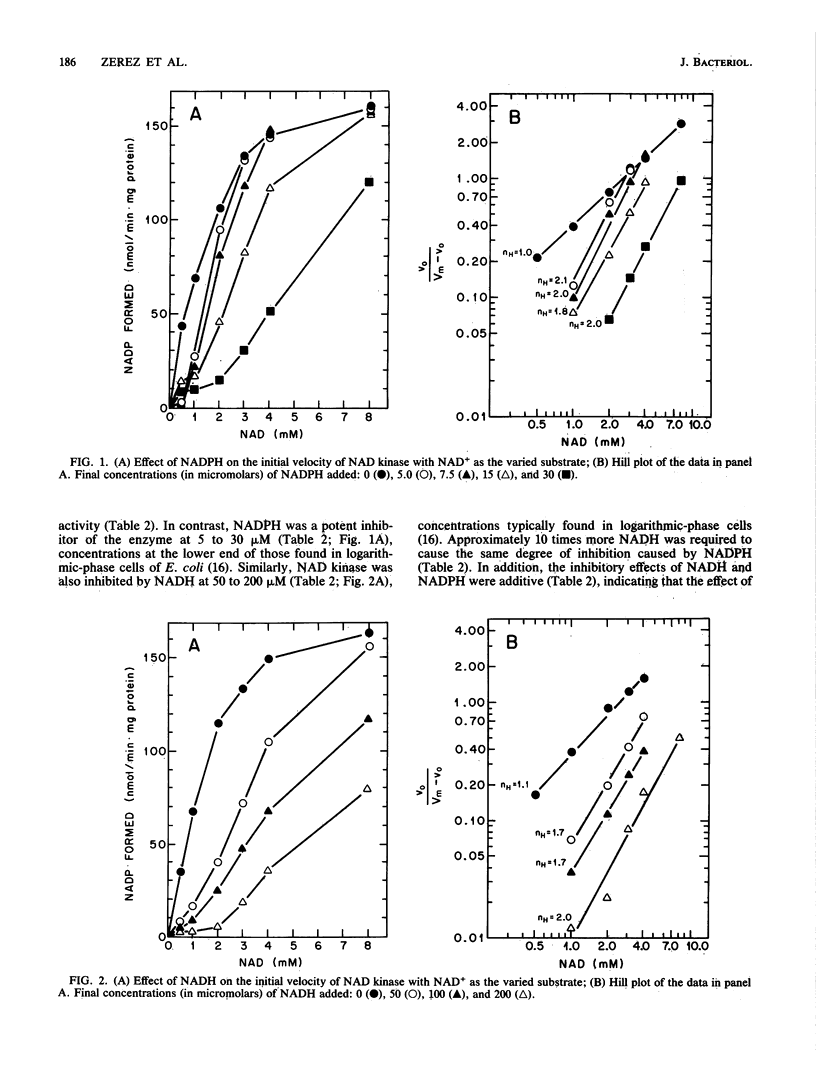
NAD kinase was purified 93-fold from Escherichia coli. The enzyme was found to have a pH optimum of 7.2 and an apparent Km for NAD+, ATP, and Mg2+ of 1.9, 2.1, and 4.1 mM, respectively. Several compounds including quinolinic acid, nicotinic acid, nicotinamide, nicotinamide mononucleotide, AMP, ADP, and NADP+ did not affect NAD kinase activity. The enzyme was not affected by changes in the adenylate energy charge. In contrast, both NADH and NADPH were potent negative modulators of the enzyme, since their presence at micromolar concentrations resulted in a pronounced sigmoidal NAD+ saturation curve. In addition, the presence of a range of concentrations of the reduced nucleotides resulted in an increase of the Hill slope (nH) to 1.7 to 2.0 with NADH and to 1.8 to 2.1 with NADPH, suggesting that NAD kinase is an allosteric enzyme. These results indicate that NAD kinase activity is regulated by the availability of ATP, NAD+, and Mg2+ and, more significantly, by changes in the NADP+/NADPH and NAD+/NADH ratios. Thus, NAD kinase probably plays a role in the regulation of NADP turnover and pool size in E. coli.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. B., von Meyenburg K. Charges of nicotinamide adenine nucleotides and adenylate energy charge as regulatory parameters of the metabolism in Escherichia coli. J Biol Chem. 1977 Jun 25;252(12):4151–4156. [PubMed] [Google Scholar]
- Andreoli A. J., Grover T., Gholson R. K., Matney T. S. Evidence for a functional pyridine nucleotide cycle in Escherichia coli. Biochim Biophys Acta. 1969 Dec 30;192(3):539–541. doi: 10.1016/0304-4165(69)90408-5. [DOI] [PubMed] [Google Scholar]
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Brunngraber E. F., Chargaff E. Nicotinamide adenine dinucleotide as substrate of the nucleotide phosphotransferase from Escherichia coli. Biochemistry. 1973 Jul 31;12(16):3012–3016. doi: 10.1021/bi00740a010. [DOI] [PubMed] [Google Scholar]
- Chung A. E. Nicotinamide adenine dinucleotide kinase from Azotobacter vinelandii. I. Purification and properties of the enzyme. J Biol Chem. 1967 Mar 25;242(6):1182–1186. [PubMed] [Google Scholar]
- Cornish-Bowden A., Koshland D. E., Jr Diagnostic uses of the Hill (Logit and Nernst) plots. J Mol Biol. 1975 Jun 25;95(2):201–212. doi: 10.1016/0022-2836(75)90390-3. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Tanaka Y., Kawashima K. Immobilization of microbial cells containing NAD-kinase. Biotechnol Bioeng. 1979 Jun;21(6):1019–1030. doi: 10.1002/bit.260210607. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundquist R., Olivera B. M. Pyridine nucleotide metabolism in Escherichia coli. I. Exponential growth. J Biol Chem. 1971 Feb 25;246(4):1107–1116. [PubMed] [Google Scholar]
- Lusk J. E., Williams R. J., Kennedy E. P. Magnesium and the growth of Escherichia coli. J Biol Chem. 1968 May 25;243(10):2618–2624. [PubMed] [Google Scholar]
- Matin A., Gottschal J. C. Influence of dilution rate on NAD(P) and NAD(P)H concentrations and ratios in a Pseudomonas sp. grown in continuous culture. J Gen Microbiol. 1976 Jun;94(2):333–341. doi: 10.1099/00221287-94-2-333. [DOI] [PubMed] [Google Scholar]
- Oka H., Field J. B. Inhibition of rat liver nicotinamide adenine dinucleotide kinase by reduced nicotinamide adenine dinucleotide phosphate. J Biol Chem. 1968 Feb 25;243(4):815–819. [PubMed] [Google Scholar]
- PARDEE A. B., YATES R. A. Pyrimidine biosynthesis in Escherichia coli. J Biol Chem. 1956 Aug;221(2):743–756. [PubMed] [Google Scholar]
- Sanwal B. D. Allosteric controls of amphilbolic pathways in bacteria. Bacteriol Rev. 1970 Mar;34(1):20–39. doi: 10.1128/br.34.1.20-39.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanwal B. D. Regulatory mechanisms involving nicotinamide adenine nucleotides as allosteric effectors. 3. Control of glucose 6-phosphate dehydrogenase. J Biol Chem. 1970 Apr 10;245(7):1626–1631. [PubMed] [Google Scholar]
- Sanwal B. D. Regulatory mechanisms involving nicotinamide adenine nucleotides as allosteric effectors. I. Control characteristics of malate dehydrogenase. J Biol Chem. 1969 Apr 10;244(7):1831–1837. [PubMed] [Google Scholar]
- Sanwal B. D., Smando R. Malic enzyme of Escherichia coli. Diversity of the effectors controlling enzyme activity. J Biol Chem. 1969 Apr 10;244(7):1817–1823. [PubMed] [Google Scholar]
- Sanwal B. D., Smando R. Malic enzyme of Escherichia coli. Possible mechanism for allosteric effects. J Biol Chem. 1969 Apr 10;244(7):1824–1830. [PubMed] [Google Scholar]
- Uchida T., Watanabe T., Kato J., Chibata I. Continuous production of NADP by immobilized Achromobacter aceris cells. Biotechnol Bioeng. 1978 Feb;20(2):255–266. doi: 10.1002/bit.260200208. [DOI] [PubMed] [Google Scholar]
- WANG T. P., KAPLAN N. O. Kinases for the synthesis of coenzyme A and triphosphopyridine nucleotide. J Biol Chem. 1954 Jan;206(1):311–325. [PubMed] [Google Scholar]
- Weitzman P. D. Reduced nicotinamide-adenine dinucleotide as an allosteric effector of citrate-synthase activity in Escherichia coli. Biochem J. 1966 Dec;101(3):44C–45C. doi: 10.1042/bj1010044c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimpenny J. W., Firth A. Levels of nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide in facultative bacteria and the effect of oxygen. J Bacteriol. 1972 Jul;111(1):24–32. doi: 10.1128/jb.111.1.24-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. A., Sanwal B. D. Regulatory mechanisms involving nicotinamide adenine nucleotides as all teric effectors. II. Control of phosphoenolpyruvate carboxykinase. J Biol Chem. 1969 Apr 10;244(7):1838–1845. [PubMed] [Google Scholar]
- Zerez C. R., Moul D. E., Andreoli A. J. NAD kinase from Bacillus licheniformis: inhibition by NADP and other properties. Arch Microbiol. 1986 May;144(4):313–316. doi: 10.1007/BF00409878. [DOI] [PubMed] [Google Scholar]



