Missense mutations in the genes encoding amyloid precursor protein (APP), presenilin 1 (PSEN1) and presenilin 2 (PSEN2) have been found to cause some forms of autosomal dominant early onset Alzheimer disease (AD). Autosomal dominant point mutations in the APP gene are associated with β‐amyloid peptide related cerebral amyloid angiopathy (CAA) and AD.1 Duplications of the APP locus on chromosome 21 have recently been reported to be associated with a phenotype similar to that caused by point mutations in the APP gene, including progressive AD and strokes and intracerebral haemorrhage (ICH) of variable frequency.2,3,4 The neuropathological findings have been consistent with a diagnosis of definite AD according to the Consortium to Establish a Registry for Alzheimer's Disease (CERAD). The most prominent feature in all cases has been severe CAA in the leptomeningeal vessels together with superficial and deep intraparenchymatous small arteries, capillaries and venules.
We have previously described detailed clinical features of a four generation Finnish family, including 14 members with progressive cognitive decline with an age of onset between 40 and 54 years.5 In addition, five patients had suffered lobar ICH. Pathological examinations of three cases revealed neuritic plaques and neurofibrillary tangles compatible with Braak stages V to VI, and also prominent CAA. Although sequencing of the APP gene failed to reveal any mutation, analysis of four single nucleotide polymorphisms located at the APP locus revealed that the affected family members harboured a common haplotype.5
We analysed the APP locus for duplications as the phenotype is similar to that found in several families with such a duplication.2,3,4 Quantitative multiplex PCR of short fluorescent fragments (QMPSF) was used to analyse samples from nine affected patients and nine healthy spouses in the family. In brief, this method is based on the simultaneous amplification of multiple short genomic sequences using dye labelled primers under quantitative conditions.2 The study was approved by the ethics committee of Oulu University Hospital (Oulu, Finland).
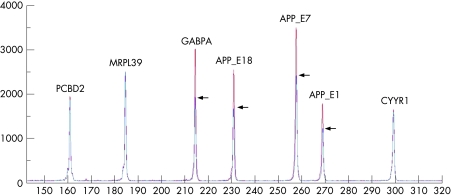
A first QMPSF assay (including three APP amplicons located in exons 1, 7 and 18) revealed that the APP and the neighbouring GAPBA genes were duplicated in two patients (fig 1). Subsequently, the QMPSF assay was performed in seven other affected relatives and nine healthy spouses blind to the clinical status of the individuals. This analysis allowed us to correctly discriminate all affected subjects from unaffected controls on the basis of the duplication pattern. The probability of obtaining this result by chance is (½)16. Subsequent QMPSF assays, including two exonic amplicons by gene, were then performed to characterise the size of the duplicated segment in the nine patients (data not shown). The duplication included the genes JAM2, ATP5J, GAPBA (centromeric to the APP locus) and APP. The first gene telomeric to the APP locus (CYYR1) was not duplicated, thus predicting a minimum size of 0.55 Mb for the duplicated segment.
Figure 1 Detection of amyloid precursor protein (APP) duplication by quantitative multiplex PCR of short fluorescent fragments (QMPSF). The electropherogram of the affected subject (in red) was superimposed on that of a normal individual (in blue) by adjusting the peaks obtained from the control amplicon PCBD2 located on chromosome 5 to the same level. The vertical axis shows fluorescence in arbitrary units and the horizontal axis indicates the size of the amplicons in base pairs. Arrows indicate heterozygous duplication of the amplicons, as detected by a 1.5 fold heightening of the corresponding peaks. This QMPSF covers four genes located at 21q21: MRPL39, GABPA, APP and CYYR1.
The pedigree harbouring the 0.55 Mb duplication presented with CAA and frequent ICH, a phenotype quite similar to that noted in other families with APP locus duplication, which included both early onset dementia and CAA. The variation in phenotype was particularly distinct within this pedigree however. Five patients had suffered one or more episodes of ICH, and hemosiderin deposits in MRI, suggesting previous cerebral microbleeds, were seen on MRI in one male patient at age 48 years, suggesting an overall frequency of 43% for haemorrhagic events. Five of the patients with ICH or hemosiderin deposits on MRI were men and the haemorrhagic events had occurred in the early stage of the disease. Only one woman had ICH and this had occurred 18 years after the first cognitive symptoms. Although a high frequency of seizures and convulsions has been reported in both French and Dutch families,3,4 epileptic seizures were not common in the Finnish family, as only two of the 14 symptomatic patients (14%) suffered from seizures, occurring in patients with haemorrhage. No clinical features of Down syndrome were seen in the patients with duplication. The duplicated region varies in size and localisation between the reported families and may not have any correlation with the phenotype. All of the Finnish patients carried the ApoE 3/3 genotype. Thus there may be other factors that contribute to the probability of ICH as well as APP locus duplication itself or the ApoE genotype.
Our observation confirms that an increase in APP gene dosage is linked to a phenotype consisting of early onset dementia and CAA with frequent ICH. The phenotype does not depend on the size of the duplicated region, but may vary between families and between the genders. The finding also further supports the hypothesis that the APP gene is located in a hotspot region of increased recombination.
Footnotes
This work was supported by the France Alzheimer Foundation (AR‐L) and the Finnish Medical Foundation (AMR).
Competing interests: None.
References
- 1.Blennow K, de Leon M J, Zetterberg H. Alzheimer's disease. Lancet 2006368387–403. [DOI] [PubMed] [Google Scholar]
- 2.Rovelet‐Lecrux A, Hannequin D, Raux G.et al APP locus duplication causes autosomal dominant early‐onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet 20063824–26. [DOI] [PubMed] [Google Scholar]
- 3.Cabrejo L, Guyant‐Maréchal L, Laquerrière A.et al Phenotype associated with APP duplication in five families. Brain 20061292966–2976. [DOI] [PubMed] [Google Scholar]
- 4.Sleegers K, Brouwers N, Gijselinck I.et al APP duplication is sufficient to cause early onset Alzheimer's dementia with cerebral amyloid angiopathy. Brain 20061292977–2983. [DOI] [PubMed] [Google Scholar]
- 5.Remes A M, Finnilä S, Mononen H.et al Hereditary dementia with intracerebral hemorrhages and cerebral amyloid angiopathy. Neurology 200463234–240. [DOI] [PubMed] [Google Scholar]