Abstract
Objective
To compare the profile of cognitive impairment in Alzheimer's disease (AD) with dementia associated with Parkinson's disease (PDD).
Methods
Neuropsychological assessment was performed in 488 patients with PDD and 488 patients with AD using the Mini‐Mental State Examination (MMSE) and the Alzheimer's Disease Assessment Scale‐cognitive subscale (ADAS‐cog). Logistic regression analysis was used to investigate whether the diagnosis could be accurately predicted from the cognitive profile. Additionally, the cognitive profiles were compared with a normative group using standardised effect sizes (Cohen's d).
Results
Diagnosis was predicted from the cognitive profile, with an overall accuracy of 74.7%. Poor performance of the AD patients on the orientation test in ADAS‐cog best discriminated between the groups, followed by poor performance of the PDD patients on the attentional task in MMSE. Both groups showed memory impairment, AD patients performing worse than PDD patients.
Conclusion
The cognitive profile in PDD differs significantly from that in AD. Performance on tests of orientation and attention are best in differentiating the groups.
Alzheimer's disease (AD) and Parkinson's disease (PD) are the most common neurodegenerative diseases in the elderly. AD is primarily a dementing disease whereas PD is mainly characterised by a movement disorder. However, dementia is common among patients with PD (PDD), with an average point prevalence of 31%1 and a cumulative prevalence close to 80%.2 In PD, dementia is associated with rapid motor3 and functional decline,4 and increased mortality.5
Cortical Lewy body pathology correlates best with dementia in PD6,7,8,9; subcortical pathology10 and AD‐type pathology11 have also been found to be associated with PDD. In addition to differences in morphological changes, AD and PDD also differ in the regional pattern of the pathology. In AD the first and most pronounced changes are found in the entorhinal cortex and parahippocampal region,12 subsequently involving neocortical areas, including the posterior association cortices.13 In contrast, in patients with PD without dementia, brainstem nuclei and other subcortical structures are initially affected.14 In PDD, limbic areas, neocortical association cortices, and the motor cortex and primary sensory cortical areas are thought to be successively involved with disease progression.15
Given the difference in the distribution and progression of pathology in AD and PDD, it is expected that their cognitive profiles would also differ.16,17 AD is characterised by memory loss emerging in the early stages of the disease,18 primarily involving learning and encoding deficits19 which are associated with medial temporal lobe pathology.20,21,22,23 As the disease progresses, deficits in language, praxis, visuospatial and executive functions gradually develop. In contrast, the cognitive deficits in the early stages of PDD are characterised by executive dysfunction, including impairment in attention24 and working memory,25,26,27 reflecting involvement of brainstem nuclei and frontal–subcortical circuits; deficits in visuoperceptual28,29,30 and visuoconstructional tasks are also frequent.31 Memory impairment is often present26,32,33,34 but whether it is primarily a consequence of frontally mediated executive deficits resulting in poor learning efficacy and retrieval, or whether involvement of limbic areas directly related to memory encoding (such as hippocampal atrophy) also contribute to memory impairment, is debated. Patients with PDD have difficulties in retrieving newly learned material, but perform better in recognition,35 indicating that executive, rather than encoding, deficits, is the underlying mechanism. Conflicting results, however, have been reported recently36,37 which could indicate that the type and mechanisms of memory deficits may vary within the PD group.32
Most studies investigating the cognitive profile of PDD patients included small samples which were not community based and thus not necessarily representative of the PD population at large. As there is evidence of interindividual heterogeneity,33 such studies may not adequately reflect the cognitive profile of patients with PDD. In order to assess the profile of cognitive deficits in PDD compared with AD in larger patient populations, we analysed the baseline cognitive data from large clinical trials conducted with the cholinesterase inhibitor rivastigmine.38,39
Methods
Patients
In all, 488 patients with PDD and 488 patients with AD involved in worldwide clinical trials of rivastigmine38,39 were included in the analyses.
Patients with AD were selected from a database of 2791 patients with mild to moderate AD. Each AD patient was individually matched to a PDD patient with regard to sex and duration of dementia. Furthermore, the AD patients were individually matched to the PDD patients for age and Mini‐Mental State Examination (MMSE). Firstly, all AD patients were reviewed for an ideal individual match on all variables. If no match was found, the age criterion was relaxed to ±2 years and the MMSE criterion to ±1 year. Patients were diagnosed with AD according to the Diagnostic and statistical manual of mental disorders, 4th edn, and NINCDS‐ADRDA (National Institute of Neurological and Communicative Diseases and Stroke‐Alzheimer's Disease and Related Disorders Association) criteria for dementia of the Alzheimer's type and had an MMSE score of 10–26, inclusive. Patients with significant physical illness, psychiatric or neurological disorders other than Alzheimer's disease, or clinically important laboratory abnormalities, including impaired renal or liver function, were excluded from the AD studies.
The PDD patients were selected from a database of 541 patients. Fifty‐three patients were excluded from the analyses because matching patients with AD could not be found or parts of the Alzheimer's Disease Assessment Scale‐cognitive subscale (ADAS‐cog) were missing. PD was diagnosed according to the clinical diagnostic criteria of the UK Parkinson's Disease Society Brain Bank40 and dementia caused by PD according to the Diagnostic and statistical manual of mental disorders, 4th edn (code 294.1).41 Patients had mild to moderately severe dementia, as defined by an MMSE score of 10–26, inclusive, with the onset of symptoms occurring at least 2 years after the diagnosis of PD. Exclusion criteria included the presence of any primary neurodegenerative disorder other than PD or other causes of dementia; a history of a major depressive episode; the presence of an active, uncontrolled seizure disorder; the presence of any disability or unstable disease unrelated to PD; and the use of a cholinesterase inhibitor or anticholinergic drugs during the 4 weeks before inclusion in the study.
The caregivers and the mentally competent patients, or their legally authorised representative, gave written informed consent. All procedures were conducted in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration, as revised in 1983.
Cognitive measures
Two composite cognitive scales administered in all trials were used for the analysis: MMSE and ADAS‐cog.42 Subtests from these scales were used to assess the profile of cognitive deficits. The “serial 7s subtraction” task from the MMSE43 was chosen as a measure of attentional control and working memory, as ADAS‐cog lacks a test of attentional control. This parameter is referred to as “attention and calculation”. The subtests “commands, constructional praxis, ideational praxis, word recall, word recognition, naming objects/fingers and orientation” from the ADAS‐cog were analysed as separate variables. As inter‐rater reliability of the ADAS‐cog subscales, which are based on the clinician's impression, is not known, these were excluded from the analyses (“comprehension, remembering test instructions, spoken language ability and word finding difficulty in spontaneous speech”).
The sign of the “attention and calculation” variable derived from the MMSE was reversed to make it compatible with ADAS‐cog scores, with higher scores indicating worse cognitive functioning.
Statistical analyses
Data were analysed using SPSS version 13.01. As most of the variables were not normally distributed, the Mann–Whitney U test was used for all single variable comparisons of the two groups. Logistic regression was used to assess the predictive power of the cognitive profile in discriminating between PDD and AD. In addition, the impact of each variable was assessed, testing for statistical significance as well as effect size using odds ratios for each predictor variable. The predictors were standardised in order to make interpretable comparisons of the odds ratios. The predictors in the regression analyses did not show multicollinearity, as none of the condition indices exceeded a threshold of 15.44
To compare the data from the AD and PDD patients with normative data, the effect sizes (Cohen's d) of the differences between the AD and PDD patients and normal controls were computed using the differences between the means divided by the pooled standard deviations, as recommended by Hedges and Olkins.45 Published normative data from 124 healthy older subjects were used for this analysis.46 The mean age of the normal group was 71.2 (SD 5.89) years; there were 65 men (52.4%), and all were Caucasian.
Results
Baseline demographic and clinical characteristics are summarised in table 1.
Table 1 Baseline demographic and background characteristics.
| Variable | PDD | AD |
|---|---|---|
| Sex (M:F) | 314:174 | 314:174 |
| Duration of dementia (y) | 2.25 (1.13) | 2.25 (1.13) |
| Age (y) | 72.63 (6.43) | 72.65 (6.43) |
| MMSE score | 19.67 (3.52) | 19.72 (3.53) |
| ADAS‐cog total score | 23.75 (10.15) | 23.30 (10.11) |
| Hoehn & Yahr stage | 2.94 (0.88) | ND |
AD, Alzheimer's disease; ADAS‐cog, Alzheimer's Disease Assessment Scale‐cognitive subscale; MMSE, Mini‐Mental State Examination; ND, not done; PDD, dementia associated with Parkinson's disease.
Values are mean (SD) or number.
There were no significant differences between the samples in terms of sex, duration of dementia, age, total MMSE or ADAS‐cog score. Almost all PDD patients, and <1% of AD subjects, had been treated with levodopa or other dopaminergic medications. Eighteen patients with AD (3.7%) and 126 patients with PDD (25.8%) had been treated with antidepressants. Thirteen AD patients (2.7%) and 127 (26%) PDD patients had received antipsychotics.
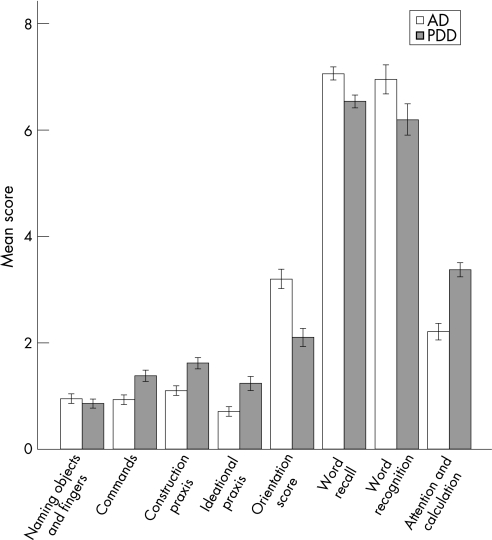
The mean and median cognitive scores as well as the results of the Mann–Whitney U test are shown in table 2 and the cognitive profile of PDD patients compared with those with AD is shown in fig 1.
Table 2 Cognitive variables in patients with Alzheimer's disease and in those with dementia associated with Parkinson's disease.
| Variable | Group | Mean | SD | Median | Mann– Whitney U | Z values | p Values |
|---|---|---|---|---|---|---|---|
| Naming objects and fingers | AD | 0.95 | 0.99 | 1 | 114177 | −1.19 | 0.235 |
| PDD | 0.86 | 0.91 | 1 | ||||
| Commands | AD | 0.93 | 1.06 | 1 | 92760 | −6.24 | <0.000 |
| PDD | 1.38 | 1.18 | 1 | ||||
| Constructional praxis | AD | 1.10 | 0.99 | 1 | 88094 | −7.38 | <0.000 |
| PDD | 1.62 | 1.14 | 1 | ||||
| Ideational praxis | AD | 0.70 | 1.03 | 0 | 96604 | −5.49 | <0.000 |
| PDD | 1.24 | 1.48 | 1 | ||||
| Orientation | AD | 3.20 | 2.02 | 3 | 81213 | −8.70 | <0.000 |
| PDD | 1.10 | 1.91 | 2 | ||||
| Word recall | AD | 7.06 | 1.43 | 7 | 94901 | −5.49 | <0.000 |
| PDD | 6.54 | 1.39 | 6.67 | ||||
| Word recognition | AD | 6.95 | 3.04 | 7 | 102725 | −3.72 | <0.000 |
| PDD | 6.20 | 3.30 | 6 | ||||
| Attention and calculation | AD | 2.21 | 1.73 | 2 | 73677 | −10.49 | <0.000 |
| PDD | 3.37 | 1.48 | 4 |
AD, Alzheimer's disease; PDD, dementia associated with Parkinson's disease.
Mann–Whitney U and p values for comparisons of the groups are given.
Figure 1 Cognitive profile of dementia associated with Parkinson's disease (PDD) compared with Alzheimer's disease (AD). The y axis represents mean raw score. Higher scores indicate worse performance. Values are mean (95% CI).
The groups differed significantly for all measures except “naming objects and fingers”. Based on the Z scores, the groups differed most on “attention and calculation”, followed by “orientation scores”. To investigate whether psychiatric drugs could have influenced the results, we performed the same analyses on patients who did not receive antidepressants or antipsychotics (PDD n = 268, AD n = 458), but this did not change the pattern of results.
ADAS‐cog variables were also compared with normative data46 by calculating effect sizes (Cohen's d) based on the differences in means following the procedure recommended by Hedges and Olkins.45 By convention, effect sizes exceeding 0.8 are considered large. All effect sizes were greater than 0.8, indicating that both patient groups showed marked deficits on all cognitive measures compared with controls. Both patient groups were most impaired at “word recall”, followed by “word recognition”, with an effect size exceeding 1.5 for both of these measures. Several of the variables showed ceiling effects for the control group, reducing the effect size scores.
A direct logistic regression analysis showed that the full model with all nine variables was statistically reliable (χ2 (8, n = 976) = 337; p<0.001), indicating that the cognitive profile distinguished between patients with AD and PDD. The variance in diagnosis accounted for was moderate, with Nagelkerke R2 = 0.39. Correct prediction of PDD was 76.8% and AD 72.5%, yielding an overall success rate of 74.7%.
Regression coefficients, Wald statistics with p values, odds ratios and 95% confidence intervals for odds ratios for each of the nine variables are shown in table 3.
Table 3 Logistic regression with diagnosis as outcome and standardised cognitive variables as predictors.
| Variable | B | Wald test | df | p Value | OR | 95% CI for OR | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Naming objects and fingers | −0.082 | 0.87 | 1 | <0.349 | 0.921 | 0.775 | 1.094 |
| Commands | 0.422 | 18.99 | 1 | <0.000 | 1.525 | 1.261 | 1.844 |
| Constructional praxis | 0.377 | 18.51 | 1 | <0.000 | 1.458 | 1.228 | 1.731 |
| Ideational praxis | 0.483 | 23.95 | 1 | <0.000 | 1.621 | 1.336 | 1.967 |
| Orientation | −0.806 | 62.84 | 1 | <0.000 | 0.446 | 0.366 | 0.545 |
| Word recall | −0.560 | 3.30 | 1 | <0.000 | 0.571 | 0.468 | 0.697 |
| Word recognition | −0.062 | 0.48 | 1 | <0.485 | 0.939 | 0.788 | 1.119 |
| Attention and calculation | 0.675 | 57.20 | 1 | <0.000 | 1.963 | 1.648 | 2.338 |
| Constant | −0.038 | 0.24 | 1 | <0.620 | 0.962 | ||
Only “naming objects and fingers” and “word recognition” were non‐significant predictors of diagnostic category. “Orientation” was the strongest predictor, with an odds ratio (OR) of 0.45, indicating a substantial reduction in the likelihood of being in the PDD group, as a consequence of a 1 point increase in Z score for the “orientation” variable. “Attention and calculation” was the second strongest predictor, with an OR of 1.96, indicating a relatively large increase in the likelihood of being in the PDD group with a 1 point increase in Z score for the “attention and calculation variable”.
Discussion
Our results confirm that patients with PDD have a significantly different cognitive profile than those with AD, supporting the hypothesis that dementia in PD is not caused by concomitant AD. The logistic regression analysis indicated that 74.7% of the patients were correctly classified as having AD or PDD, based on the cognitive profile. Based on estimates of odds ratios from the logistic regression analysis, the strongest predictor of diagnostic category was the variable “orientation” followed by “attention and calculation”.
The results further demonstrated the specific pattern of cognitive impairment in PDD compared with AD. The markedly impaired performance on attention and calculation (the serial 7s test) in PDD compared with AD indicates a more pronounced attentional deficit in PDD, as has been reported previously.47 The more severe deficits in PDD compared with AD in cholinergic pathways subserving frontal–subcortical circuits48,49 may be the underlying mechanism for the prominent attentional deficits in PDD.
Patients with AD showed more severe memory impairment than those with PDD. On the verbal memory tasks in the ADAS‐cog, however, both groups were clearly impaired relative to a normal control group, with very large effect sizes. The hypothesis that the memory deficit in PD and PDD is mainly a result of a retrieval rather than an encoding deficit35 was not answered by this study. Patients with PDD and patients with AD were clearly impaired on the measure of verbal recognition memory. However, the ADAS‐cog recognition memory test may be problematic as it is not based on the same word list as the recall memory test, but rather on a single learning trial of a new word list. Thus other memory processes may explain the differences between the repeated list based on a recall format and the recognition list that was presented only once.
The finding that the PDD patients were more impaired on the praxis items (commands, constructional praxis and ideational praxis) on the ADAS‐cog is difficult to interpret, given the impact of motor disability on these tasks.
Our study had several limitations which may have influenced the results. Firstly, as patients were recruited for clinical trials, they may not accurately represent the overall population of AD and PDD, especially those with severe dementia, as such patients were excluded from the study. Matching the patients on severity of dementia can be problematic, given that there is no established method for such matching when the issue at hand is differences in cognitive profile between two patients groups. However, the MMSE represents a well established approach to this problem, and the total ADAS‐cog score was the same in both groups.
Secondly, the analyses of cognitive profiles were based on composite cognitive scales; detailed neuropsychological testing was not employed and hence a more comprehensive comparison of cognitive functions was not possible. Ceiling effects may have affected some of the subtests, indicated by a median score of 0 for some of the variables and an overall skewness of some of the distributions. Also, the serial 7s “attention and calculation” task of the MMSE was one of the major predictors of diagnostic category but it was part of the MMSE score used for matching the groups on severity of dementia. This may have led to an underestimation of the difference between the groups for this variable. However, as ADAS‐cog lacks a measure of attentional control, the variable was included to give a more complete picture of the cognitive profiles. The sum of these issues, however, strengthens the conclusion that AD and PDD have different cognitive profiles as differences were found even with a possibly deflated group difference on the serial 7s task, the rather crude cognitive testing and with reduced variance as a consequence of ceiling effects.
Thirdly, there were differences in drug treatment between the groups, including the used of antidepressants and antipsychotics, but excluding patients receiving medications did not change the pattern of differences on the cognitive variables. All PDD, but very few AD, patients were receiving dopaminergic medications. These medications may influence cognitive functions in a complex way. In one study, for example, executive functions, as measured by the Wisconsin Card Sorting Test, were impaired by levodopa, while response time on several tests improved, but accuracy did not.50 Conflicting results have been reported on the effect of levodopa on cognitive functions in other studies, some reporting improvement, some no change and some worsening.
Finally, the cross sectional design and lack of neuropathological confirmation of the diagnoses may limit the validity of the findings to some extent.
The major strength of the study is the large number of patients included; this is by far the largest study ever published comparing AD with PDD. The patients in the two groups were individually matched with regard to age, sex, duration of dementia and overall cognitive impairment, and they were all studied using the same cognitive scales which enabled a direct comparison.
In conclusion, we found differential cognitive profiles in patients with PDD and AD, strongly supporting the notion that dementia in PDD is not caused by an AD‐type pathology but rather by pathophysiological mechanisms specifically associated with PD.
Acknowledgements
Novartis Pharma sponsored the original studies and provided the data for the present study. Kolbjorn Bronnick was financed by the Research Council of Norway.
Abbreviations
AD - Alzheimer's disease
ADAS‐cog - Alzheimer's Disease Assessment Scale‐cognitive subscale
MMSE - Mini‐Mental State Examination
PD - Parkinson's disease
PDD - dementia associated with Parkinson's disease
Footnotes
Competing interests: None.
References
- 1.Aarsland D, Zaccai J C B, Brayne C. A systematic review of prevalence studies of dementia in Parkinson's disease. Mov Disord 2005201255–1263. [DOI] [PubMed] [Google Scholar]
- 2.Aarsland D, Andersen K, Larsen J P.et al Prevalence and characteristics of dementia in Parkinson disease: an 8‐year prospective study. Arch Neurol 200360387–392. [DOI] [PubMed] [Google Scholar]
- 3.Louis E D, Tang M X, Cote L.et al Progression of parkinsonian signs in Parkinson's disease. Arch Neurol 199956334–337. [DOI] [PubMed] [Google Scholar]
- 4.Marras C, Rochon P, Lang A E. Predicting motor decline and disability in Parkinson's disease. Arch Neurol 2002591724–1728. [DOI] [PubMed] [Google Scholar]
- 5.Levy G, Tang M X, Louis E D.et al The association of incident dementia with mortality in PD. Neurology 2002591708–1713. [DOI] [PubMed] [Google Scholar]
- 6.Kosaka K, Tsuchiya K, Yoshimura M. Lewy body disease with and without dementia: a clinicopathological study of 35 cases. Clin Neuropathol 19887299–305. [PubMed] [Google Scholar]
- 7.Mattila P M, Roytta M, Torikka H.et al Cortical Lewy bodies and Alzheimer‐type changes in patients with Parkinson's disease. Acta Neuropathol (Berl) 199895576–582. [DOI] [PubMed] [Google Scholar]
- 8.Mattila P M, Rinne J O, Helenius H.et al Alpha‐synuclein‐immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson's disease. Acta Neuropathol (Berl) 2000100285–290. [DOI] [PubMed] [Google Scholar]
- 9.Hurtig H I, Trojanowski J Q, Galvin J.et al Alpha‐synuclein cortical Lewy bodies correlate with dementia in Parkinson's disease. Neurology 2000541916–1921. [DOI] [PubMed] [Google Scholar]
- 10.Rinne J O, Rummukainen J, Paljarvi L.et al Dementia in Parkinson's disease is related to neuronal loss in the medial substantia nigra. Ann Neurol 19892647–50. [DOI] [PubMed] [Google Scholar]
- 11.Jellinger K A, Seppi K, Wenning G K.et al Impact of coexistent Alzheimer pathology on the natural history of Parkinson's disease. J Neural Transm 2002109329–339. [DOI] [PubMed] [Google Scholar]
- 12.Busatto G F, Garrido G E, Almeida O P.et al A voxel‐based morphometry study of temporal lobe gray matter reductions in Alzheimer's disease. Neurobiol Aging 200324221–231. [DOI] [PubMed] [Google Scholar]
- 13.Karas G B, Scheltens P, Rombouts S A.et al Global and local gray matter loss in mild cognitive impairment and Alzheimer's disease. Neuroimage 200423708–716. [DOI] [PubMed] [Google Scholar]
- 14.Braak H, Del Tredici K, Rub U.et al Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 200324197–211. [DOI] [PubMed] [Google Scholar]
- 15.Braak H, Rub U, Jansen Steur E N.et al Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 2005641404–1410. [DOI] [PubMed] [Google Scholar]
- 16.Janvin C C, Larsen J P, Salmon D P.et al Cognitive profiles of individual patients with Parkinson's disease and dementia: Comparison with dementia with lewy bodies and Alzheimer's disease. Mov Disord 200621337–342. [DOI] [PubMed] [Google Scholar]
- 17.Stern Y, Tang M X, Jacobs D M.et al Prospective comparative study of the evolution of probable Alzheimer's disease and Parkinson's disease dementia. J Int Neuropsychol Soc 19984279–284. [PubMed] [Google Scholar]
- 18.Backman L, Jones S, Berger A K.et al Cognitive impairment in preclinical Alzheimer's disease: a meta‐analysis. Neuropsychology 200519520–531. [DOI] [PubMed] [Google Scholar]
- 19.Greene J D, Baddeley A D, Hodges J R. Analysis of the episodic memory deficit in early Alzheimer's disease: evidence from the doors and people test. Neuropsychologia 199634537–551. [DOI] [PubMed] [Google Scholar]
- 20.Jobst K A, Smith A D, Szatmari M.et al Detection in life of confirmed Alzheimer's disease using a simple measurement of medial temporal lobe atrophy by computed tomography. Lancet 19923401179–1183. [DOI] [PubMed] [Google Scholar]
- 21.Nagy Z, Jobst K A, Esiri M M.et al Hippocampal pathology reflects memory deficit and brain imaging measurements in Alzheimer's disease: clinicopathologic correlations using three sets of pathologic diagnostic criteria. Dementia 1996776–81. [DOI] [PubMed] [Google Scholar]
- 22.Convit A, De Leon M J, Tarshish C.et al Specific hippocampal volume reductions in individuals at risk for Alzheimer's disease. Neurobiol Aging 199718131–138. [DOI] [PubMed] [Google Scholar]
- 23.Mori E, Yoneda Y, Yamashita H.et al Medial temporal structures relate to memory impairment in Alzheimer's disease: an MRI volumetric study. J Neurol Neurosurg Psychiatry 199763214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owen A M, James M, Leigh P N.et al Fronto‐striatal cognitive deficits at different stages of Parkinson's disease. Brain 1992115(Pt 6)1727–1751. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert B, Belleville S, Bherer L.et al Study of verbal working memory in patients with Parkinson's disease. Neuropsychology 200519106–114. [DOI] [PubMed] [Google Scholar]
- 26.Muslimovic D, Post B, Speelman J D.et al Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 2005651239–1245. [DOI] [PubMed] [Google Scholar]
- 27.Bublak P, Muller U, Gron G.et al Manipulation of working memory information is impaired in Parkinson's disease and related to working memory capacity. Neuropsychology 200216577–590. [DOI] [PubMed] [Google Scholar]
- 28.Johnson A M, Almeida Q J, Stough C.et al Visual inspection time in Parkinson's disease: deficits in early stages of cognitive processing. Neuropsychologia 200442577–583. [DOI] [PubMed] [Google Scholar]
- 29.Laatu S, Revonsuo A, Pihko L.et al Visual object recognition deficits in early Parkinson's disease. Parkinsonism Relat Disord 200410227–233. [DOI] [PubMed] [Google Scholar]
- 30.Montse A, Pere V, Carme J.et al Visuospatial deficits in Parkinson's disease assessed by judgment of line orientation test: error analyses and practice effects. J Clin Exp Neuropsychol 200123592–598. [DOI] [PubMed] [Google Scholar]
- 31.Uc E Y, Rizzo M, Anderson S W.et al Visual dysfunction in Parkinson disease without dementia. Neurology 2005651907–1913. [DOI] [PubMed] [Google Scholar]
- 32.Weintraub D, Moberg P J, Culbertson W C.et al Evidence for impaired encoding and retrieval memory profiles in Parkinson disease. Cogn Behav Neurol 200417195–200. [PubMed] [Google Scholar]
- 33.Filoteo J V, Rilling L M, Cole B.et al Variable memory profiles in Parkinson's disease. J Clin Exp Neuropsychol 199719878–888. [DOI] [PubMed] [Google Scholar]
- 34.Foltynie T, Brayne C E, Robbins T W.et al The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPaIGN study. Brain 2004127(Pt 3)550–560. [DOI] [PubMed] [Google Scholar]
- 35.Breen E K. Recall and recognition memory in Parkinson's disease. Cortex 19932991–102. [DOI] [PubMed] [Google Scholar]
- 36.Higginson C I, Wheelock V L, Carroll K E.et al Recognition memory in Parkinson's disease with and without dementia: evidence inconsistent with the retrieval deficit hypothesis. J Clin Exp Neuropsychol 200527516–528. [DOI] [PubMed] [Google Scholar]
- 37.Whittington C J, Podd J, Kan M M. Recognition memory impairment in Parkinson's disease: power and meta‐analyses. Neuropsychology 200014233–246. [DOI] [PubMed] [Google Scholar]
- 38.Emre M, Aarsland D, Albanese A.et al Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med 20043512509–2518. [DOI] [PubMed] [Google Scholar]
- 39.Rosler M, Anand R, Cicin‐Sain A.et al Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomized controlled trial. BMJ 1999318633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes A J, Daniel S E, Kilford L.et al Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 199255181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.American Psychiatric Association Diagnostic and statistical manual of mental disorders, 4th edn, DSM‐IV. Washington DC: American Psychiatric Association, 1994
- 42.Rosen W G, Mohs R C, Davis K L. A new rating scale for Alzheimer's disease. Am J Psychiatry 19841411356–1364. [DOI] [PubMed] [Google Scholar]
- 43.Folstein M F, Folstein S E, McHugh P R. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 197512189–198. [DOI] [PubMed] [Google Scholar]
- 44.Hair J F, Anderson R E, Tatham R L.et alMultivariate data analysis. New Jersey: Prentice Hall, 1998
- 45.Hedges L V, Olkin I.Statistical methods for meta‐analysis. Orlando, Florida: Academic Press, 1985
- 46.Graham D P, Cully J A, Snow A L.et al The Alzheimer's disease assessment scale—Cognitive subscale. Normative data for older control adults. Alzheimer Dis Assoc Disord 200418236–240. [PubMed] [Google Scholar]
- 47.Tamura I, Kikuchi S, Otsuki M.et al Deficits of working memory during mental calculation in patients with Parkinson's disease. J Neurol Sci 200320919–23. [DOI] [PubMed] [Google Scholar]
- 48.Bohnen N I, Kaufer D I, Hendrickson R.et al Cognitive correlates of cortical cholinergic denervation in Parkinson's disease and parkinsonian dementia. J Neurol 2006253242–247. [DOI] [PubMed] [Google Scholar]
- 49.Bohnen N I, Kaufer D I, Ivanco L S.et al Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease. Arch Neurol 2003601745–1748. [DOI] [PubMed] [Google Scholar]
- 50.Kulisevsky J, Avila A, Barbanoj M.et al Acute effects of levodopa on neuropsychological performance in stable and fluctuating Parkinson's disease patients at different levodopa plasma levels. Brain 19961192121–2132. [DOI] [PubMed] [Google Scholar]