Abstract
Objective
To evaluate the efficacy and safety of rapidly titrated rivastigmine administered twice (BID) or three times (TID) daily in patients with mild to moderate Alzheimer's disease (AD).
Methods
This was a 26 week international, randomised, double blind, placebo controlled study in which 678 patients with probable AD received placebo or rivastigmine 2–12 mg/day BID or TID. Primary outcome measures included the cognitive subscale of the AD Assessment Scale (ADAS‐cog) and categorical analysis of the Clinician Interview Based Impression of Change incorporating caregiver information (CIBIC‐Plus). Secondary outcomes were the CIBIC‐Plus change from baseline, Progressive Deterioration Scale, ADAS‐cogA, Mini‐Mental State Examination and Global Deterioration Scale.
Results
At week 26, mean rivastigmine dose was 9.6 (2.76) mg/day in the TID group and 8.9 (2.93) mg/day in the BID group. Mean ADAS‐cog changes from baseline in the TID and BID rivastigmine treated groups were −0.2 (SD 7.3) and 1.2 (SD 7.2) versus 2.8 (SD 7.2) for the placebo group (p<0.05). Differences between rivastigmine TID and placebo on the CIBIC‐Plus categorical responder analysis were significant (31% vs 19%; p<0.05, intention to treat). No significant differences were seen between BID and placebo for this outcome measure. Adverse events were predominantly gastrointestinal, occurring mainly during dose titration. Withdrawal because of adverse events accounted for 17% of BID, 11% of TID and 9% of placebo patients.
Conclusions
Rivastigmine administered as a BID or TID regimen significantly benefited cognitive, function and global performances in AD patients. The TID regimen showed a tendency for superior tolerability and permitted titration to higher doses, an outcome that is significant as the efficacy of rivastigmine is dose related.
Alzheimer's disease (AD) is characterised by a loss of cholinergic neurons and their cortical projections from the nucleus basalis and associated areas in the basal forebrain.1 Progressive deterioration of the widespread and dense cholinergic innervation of the human cerebral cortex contributes to the symptoms of AD and is associated with decreased levels of the neurotransmitter acetylcholine (ACh). Acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) appear to be simultaneously active in the synaptic hydrolysis of ACh, terminating its neurotransmitter action, and co‐regulating ACh levels.2 The use of cholinesterase inhibitors (ChE‐Is) has been directed at increasing and maintaining synaptic ACh to improve cholinergic neurotransmission.
Three regulatory approved ChE‐Is (rivastigmine, donepezil and galantamine) are widely used for the symptomatic treatment of AD. These drugs have demonstrated efficacy in treating cognitive and global functioning, while stabilising functional abilities, over at least 6 months during clinical trials in patients with mild to moderate AD.3,4 Although the currently used ChE‐Is have the same treatment indication, they differ pharmacologically.5 Rivastigmine induces a slowly reversible inhibition of both AChE and BuChE that is sustained for at least 12 months of repeated administration.6 Metabolism of rivastigmine occurs by its target enzymes (AChE and BuChE), independent of hepatic drug metabolising cytochrome enzymes. Its pharmacodynamic half life is 1.6 h following a 6 mg dose,7 although both AChE and BuChE activity are inhibited for about 8–10 h, with maximum inhibition of about 60% 5 h after dosing.8
Previous placebo controlled, randomised controlled trials (RCTs) with rivastigmine clearly demonstrated a dose–response relationship with best efficacy reported for doses between 6 and 12 mg daily.9,10 However, too rapid titration to optimal recommended doses can be associated with intolerance, particularly nausea and vomiting, which are attributed to centrally mediated cholinergic effects.11,12 Clinically, 4 weekly titration on a twice daily (BID) dosing basis with 3 mg dose increments is recommended to achieve a therapeutic response, although titration with smaller increments has been suggested to minimise side effects.13 To allow for more rapid titration, three times daily (TID) dosing, which might be associated with lower peak plasma levels, could be a useful therapeutic approach.14
The aim of this study was to evaluate the safety and efficacy of rapidly titrated rivastigmine BID or TID for 26 weeks, in patients with mild to moderately severe AD.
Methods
Patients
All patients were at least 50 years old and met the criteria for AD, as described in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition,15 and in accordance with criteria for “probable” AD of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA).16 Inclusion criteria also specified that patients were community dwelling with entry scores of 10–26 on the Mini‐Mental State Examination (MMSE).17 Recruitment was by referral to the research centre, advertising or from those known to the investigators at the participating research centre. Each patient had a responsible caregiver and, together with their caregiver, provided written informed consent according to the procedures of the ethics review board for each centre.
Patients with controlled concomitant diseases such as hypertension, non‐insulin dependent diabetes and arthritis were allowed to enter the study. Individuals with severe and unstable cardiac disease, severe obstructive pulmonary disease or other life threatening conditions were excluded. Anticholinergic drugs, health food supplements containing ACh precursors, putative memory enhancers and insulin were prohibited. All psychotropic drugs were prohibited, with the exception of chloral hydrate, short acting benzodiazepines and haloperidol, for not more than 3 days in succession and not less than 72 h before any efficacy assessment.
Study design
This 26 week, randomised, double blind, placebo controlled, parallel group study was conducted at 37 centres in Australia, Canada, Ireland, Italy, South Africa and the UK. Following screening and baseline evaluations, eligible patients were randomly assigned to one of three treatment groups. Eligible patients were randomised on a 1:1:1 basis to rivastigmine BID or TID dose regimen with a range of daily dosing of 2–12 mg/day, or matching placebo. Capsules containing rivastigmine and placebo were identical and the number taken was the same at each dose in all groups. Blinding was maintained until the last patient had completed the study. There were no interim analyses.
During the initial dose titration phase, study medication was increased from a starting dose of 2 mg/day. The dose was increased at weekly intervals in 1 mg/day steps until reaching the maximum tolerated dose. The titration phase lasted 10 days to 12 weeks, depending on the highest tolerated dose attained by the individual patient. Patients unable to tolerate 2 mg/day by day 10 were withdrawn from the study. Tolerability could be optimised by maintaining a dose level for periods of up to 2 weeks.
Having determined maximal dose tolerability, all patients then entered a maintenance phase for the remainder of the 26 week study. During the maintenance phase, further dose increases or decreases within the prescribed range were permitted at the discretion of the investigator although this was optional. Patients were instructed to take their study medication with or shortly after food. Administration of each dose level was achieved using different combinations of active medication and placebo so that the appropriate daily dose was presented as two capsules TID.
Trial procedures were in accordance with the ethical standards of the institutional committees on human experimentation and with the Helsinki Declaration. Conducted between 1994 and 1996, this study was overseen by an independent international safety monitoring board that reviewed the safety data of all patients enrolled in the study on an ongoing basis.
Outcome measures
The primary efficacy variables were the cognitive subscale of the Alzheimer's Disease Assessment Scale (ADAS‐cog)18 and categorical analysis of the Clinician Interview Based Impression of Change incorporating caregiver information scale (CIBIC‐Plus).19 Table 1 summarises the instruments used,17,18,19,20,21 including the secondary outcome measures. Efficacy evaluations were performed at baseline, weeks 12, 18 and 26 (or at early termination).
Table 1 Efficacy outcome measures.
| Outcome measure | Symptoms and domains | Scores, interpretation |
|---|---|---|
| Primary | ||
| 11‐item ADAS‐cog | Memory, language, praxis, orientation | Total score range of 0–70, where a decreasing score indicates improvement in cognitive function |
| CIBIC‐Plus | Overall global assessment of patient response | 7 point Likert scale, where 1 = markedly improved, and 7 = markedly worsened |
| Secondary | ||
| ADAS‐cogA | ADAS‐cog with an added item of attention (concentration/distractibility) | Total score range of 0–75, where a decreasing score indicates improvement in cognitive function |
| CIBIC‐Plus | Overall global assessment of change relative to baseline | 7 point Likert scale, where 1 = markedly improved, and 7 = markedly worsened |
| PDS | Activities of daily living | 29 item scale scored on a visual analogue scale of 0–100, where an increase in score indicates improvement in the patient's ability to perform activities of daily living |
| MMSE | Recent memory, attention, concentration, naming, repetition, comprehension and ability to formulate a sentence | 10 item assessment, with a range of 0−30 points with higher score representing better cognitive function |
| GDS | Overall staging of AD severity | 7 stage scale, where a higher stage indicates more advanced AD |
Safety evaluations included physical examinations, electrocardiography, monitoring vital signs and laboratory testing weekly for the first 12 weeks then every 2 weeks thereafter. Adverse events were defined as any sign, symptom, syndrome or disease that occurred for the first time after baseline, or worsened after baseline, whether or not they were considered treatment related. All adverse events were recorded using the Novartis Medical Terminology Thesaurus (a modified version of the WHO adverse reaction terminology dictionary).
Statistical methods
The study sample size was determined on the basis of an estimated 3.0 point difference between rivastigmine administered BID and placebo on the ADAS‐cog, an estimated 0.4 point difference between BID and placebo on the CIBIC‐Plus and an increased proportion of responders with CIBIC‐Plus ratings of >4 of 20% within the BID rivastigmine group (35% rivastigmine vs 15% placebo). Sample sizes of 192 per group were required. For practical reasons the sample size was chosen as 200 (intention to treat (ITT) population). An individual power of 90% guaranteed protection of the global power in view of the requirement that both ADAS‐cog and CIBIC‐Plus analyses should be significant at the 0.0499 level.
All patients who received at least one dose of study medication and who had a subsequent safety evaluation were included in the safety population. Efficacy populations were: classical ITT, traditional last observation carried forward (LOCF) and traditional observed cases (OC). The ITT population included all randomised patients, whether or not they received treatment. In the case of missing assessments, a retrieved drop‐out assessment was used; if there was no retrieved drop‐out assessment, the last prior observation available was carried forward as an imputed value. The LOCF population comprised randomised patients with at least one evaluation while being treated for whom the immediately preceding assessment was imputed to subsequently missing evaluations or data. The OC population comprised all randomised patients with an evaluation made while on study drug at all of the designated assessment times. No imputations were used for the OC analyses. The primary confirmatory analysis was based on the change from baseline at week 26 in the ITT population.
Analyses of primary efficacy variables were performed at baseline and at weeks 12, 18 and 26 on all populations. ADAS‐cog data were analysed using a two way treatment by centre analysis of covariance and variance (SAS type III analysis) on changes from baseline for each time point, using the baseline score as a covariate. In addition, a categorical analysis was performed for the ADAS‐cog to determine the proportions of patients showing at least a 4 point score at 26 weeks, with Mantel‐Haenszel blocking for centre. A categorical “CIBIC‐Plus improvers” analysis was performed to determine proportions showing improvement versus those showing no change or worsening, with Mantel‐Haenszel blocking for centre. In addition, changes from baseline on the CIBIC‐Plus were analysed using a two way analysis of variance on ratings (SAS type III analysis). Analyses of secondary efficacy variables were performed on the ITT and LOCF populations at week 26 only. For the Progressive Deterioration Scale (PDS) and ADAS‐cogA, analyses of covariance on changes from baseline to week 26 were performed. Post hoc Cohen's D effect sizes were calculated at each visit for the ADAS‐cog and CIBIC‐Plus by dividing mean differences by pooled standard deviations. These effect sizes are a useful metric for estimating the magnitude of the intervention, allowing comparisons between the BID, TID and placebo treatment arms.
Comparisons with placebo were two tailed with the critical significance level set at p<0.05. In order to control for multiplicity in the analyses of efficacy data, the primary comparison was specified as rivastigmine administered BID against placebo. If this test was statistically significant at the 0.05 level, then the rivastigmine administered TID against placebo was tested at the 0.05 level subsequently. As both primary efficacy variables were required to be significant, no further correction of the size of the tests for the multiplicity of variables was required.
Safety analyses used an analysis of variance for vital signs, laboratory data and electrocardiograms, and Fisher's exact test for the occurrence of abnormalities on physical examinations, electrocardiograms, vital signs, laboratory tests and adverse events.
Results
Patients
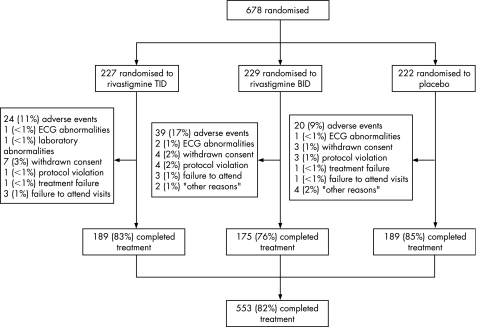
Figure 1 demonstrates patient flow through the study. Of 788 screened patients, 678 were randomised to treatment: 227 in the rivastigmine TID group, 229 in the rivastigmine BID group and 222 in the placebo group. The safety population comprised 227 patients in the rivastigmine TID group, 228 patients in the BID group and 222 in the placebo group. A total of 125 patients (18%) withdrew prematurely from the study: 38 (17%) in the rivastigmine TID group, 54 (24%) in the rivastigmine BID group and 33 (15%) in the placebo group (fig 1).
Figure 1 Patient disposition. BID, twice daily; TID, three times daily.
As seen in table 2, baseline demographic variables and disease characteristics were similar across treatment groups. There were no statistically significant differences between the groups at baseline. Most patients reported current medical conditions at baseline: 77% of patients in the rivastigmine TID group, 71% of the rivastigmine BID group and 74% of the placebo group. The most common concurrent conditions were cardiovascular disorders (hypertension) and musculoskeletal disorders (arthritis). During the study, 85%, 84% and 79% of patients receiving rivastigmine TID, rivastigmine BID or placebo, respectively, received concomitant medications. Those most commonly used during the study were drugs acting on the nervous (44%, 44% and 42%, respectively) and cardiovascular (38%, 31% and 32%, respectively) systems.
Table 2 Baseline demographics and disease characteristics (all randomised patients).
| Rivastigmine TID (n = 227) | Rivastigmine BID (n = 229) | Placebo (n = 222) | |
|---|---|---|---|
| Age (y) | 71.4 (7.9) | 71.0 (8.2) | 71.7 (8.7) |
| Sex (% M:F) | 40:60 | 43:57 | 40:60 |
| Height (cm) | 163.5 (10.7) | 164.2 (10.7) | 163.5 (10.3) |
| Weight (kg) | 65.9 (12.9) | 66.7 (12.2) | 65.9 (12.3) |
| Duration of dementia (months) | 38.4 (24.5) | 40.6 (31.2) | 39.7 (28.2) |
| Disease severity† (% patients) | |||
| Mild | 43 | 45 | 45 |
| Moderate | 55 | 53 | 52 |
| Severe | 3 | 2 | 3 |
| Mean MMSE score | 18.3 (4.5) | 18.8 (4.6) | 18.7 (4.6) |
| Mean GDS score | 4.1 (0.8) | 4.0 (0.9) | 4.1 (0.9) |
| Mean ADAS‐cog score‡ | 28.1 (12.5) | 27.7 (12.3) | 28.5 (12.3) |
ADAS‐cog, cognitive subscale of the Alzheimer's Disease Assessment Scale18; BID, twice daily; GDS, Global Deterioration Scale21; MMSE, Mini‐Mental State Examination17; TID, three times daily.
Values are mean (SD) or percentage of patients.
†Disease severity according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA).16
‡Mean ADAS‐cog score based on the intention to treat population.
Efficacy
By week 12, 60%, 43% and 88% of patients in the TID, BID and placebo groups, respectively, had reached their maximum possible dose level (12 mg/day). There was little further change in the numbers of patients with changes in the maintenance phase from their maximum possible dose level during the titration. By week 26, the mean daily dose of rivastigmine was 9.6 (2.76) mg in the TID regimen group and 8.9 (2.93) mg in the BID regimen group. At least 9 mg/day was being taken by 71% and 60% of all patients completing the study, respectively, at this time point. Patients who ingested at least 70% of the prescribed study medication were defined as treatment compliant; compliance rates were 99% for the TID group, 98% for the BID group and 99% for the placebo group.
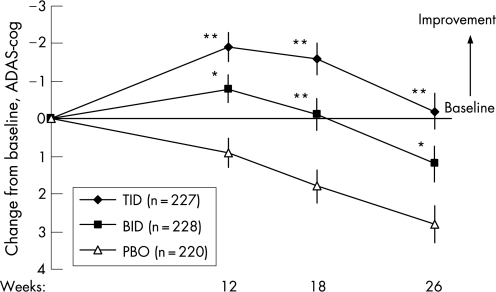
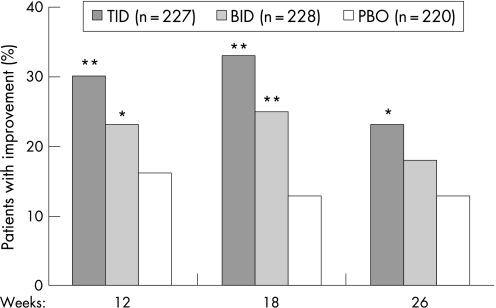
Mean ADAS‐cog changes from baseline demonstrated significant benefits in both rivastigmine groups, compared with placebo, at weeks 12, 18 and 26 (fig 2, table 3). At week 26, mean differences between rivastigmine and placebo were 2.9 in the TID group (p<0.001) and 1.6 in the BID group (p = 0.019). The magnitude of changes observed in the LOCF and OC populations were generally similar to those in the ITT population but in the OC population the BID treatment arm did not achieve significance compared with placebo at week 26 (p<0.100). Figure 3 summarises patient improvement on the ADAS‐cog during weeks 12–26 in the ITT population. Similar results were also obtained with the LOCF and OC analyses (data not shown). As seen in fig 2, in the ITT population the mean ADAS‐cog ratings were significantly better at all evaluation time points in the rivastigmine TID and BID groups compared with placebo.
Figure 2 Changes from baseline on the cognitive subscale of the Alzheimer's Disease Assessment Scale (ADAS‐cog) in the intention to treat population administered rivastigmine twice (BID) or three time (TID) daily, or placebo (PBO). *p<0.05, **p<0.001 vs placebo. p Values based on pairwise t tests using pooled error term from ANCOVA/ANOVA (SAS Type III analysis). Values are mean (SEM).
Table 3 Mean changes from baseline on efficacy variables.
| Variable | Rivastigmine TID | Rivastigmine BID | Placebo |
|---|---|---|---|
| Primary | |||
| ADAS‐cog | |||
| ITT population | 277 | 228 | 220 |
| n | |||
| Baseline score | 28.1 (12.5) | 27.7 (12.3) | 28.5 (12.3) |
| Week 26 change | −0.2 (7.3)** | 1.2 (7.2)* | 2.8 (7.2) |
| LOCF population | |||
| n | 209 | 199 | 208 |
| Baseline score | 28.3 (12.2) | 27.7 (12.3) | 28.5 (12.2) |
| Week 26 change | −0.7 (6.9)** | 0.8 (6.9)* | 2.7 (6.8) |
| OC population | |||
| n | 180 | 173 | 183 |
| Baseline score | 27.9 (11.8) | 28.6 (12.1) | 27.7 (11.9) |
| Week 26 change | −0.9 (6.8)** | 0.9 (7.0) | 2.1 (6.8) |
| CIBIC‐Plus | |||
| ITT population | |||
| n | 222 | 222 | 216 |
| Baseline score | – | – | – |
| Week 26 change | 3.9 (1.3)** | 4.1 (1.3)* | 4.5 (1.3) |
| LOCF population | |||
| n | 206 | 198 | 205 |
| Baseline score | – | – | – |
| Week 26 change | 3.9 (1.2)** | 4.1 (1.2)* | 4.5 (1.2) |
| OC population | |||
| n | 177 | 167 | 179 |
| Baseline score | – | – | – |
| Week 26 change | 3.9 (1.2)** | 4.1 (1.2)* | 4.4 (1.2) |
| Secondary | |||
| ADAS‐cogA | |||
| ITT population | |||
| n | 227 | 228 | 220 |
| Baseline score | 29.1 (13.1) | 28.6 (13.0) | 29.4 (13.0) |
| Week 26 change | −0.1 (7.9)** | 1.5 (7.8)* | 3.2 (7.8) |
| LOCF population | |||
| n | 209 | 199 | 208 |
| Baseline score | 29.2 (12.9) | 28.5 (13.0) | 29.4 (12.8) |
| Week 26 change | −0.6 (7.5)** | 1.0 (7.5)* | 3.1 (7.4) |
| PDS | |||
| ITT population | |||
| n | 225 | 227 | 221 |
| Baseline score | 49.2 (19.8) | 48.7 (19.5) | 49.0 (19.6) |
| Week 26 change | −1.5 (11.3)** | −2.6 (11.1)* | −4.9 (11.2) |
| LOCF population | |||
| n | 207 | 195 | 209 |
| Baseline score | 49.0 (19.6) | 48.6 (19.7) | 48.9 (19.4) |
| Week 26 change | −1.0 (11.4)** | −2.3 (11.5)* | −4.7 (11.3) |
| GDS | |||
| ITT population | |||
| n | 227 | 229 | 222 |
| Baseline score | 4.1 (0.9) | 4.0 (0.9) | 4.1 (0.9) |
| Week 26 change | 0.0 (0.7)* | −0.2 (0.7) | −0.3 (0.7) |
| LOCF population | |||
| n | 195 | 188 | 202 |
| Baseline score | 4.1 (0.9) | 4.0 (0.9) | 4.1 (0.9) |
| Week 26 change | −0.0 (0.7)* | −0.1 (0.7) | −0.3 (0.7) |
| MMSE | |||
| ITT population | |||
| n | 227 | 227 | 220 |
| Baseline score | 18.1 (4.7) | 18.8 (4.7) | 18.6 (4.7) |
| Week 26 change | 0.3 (3.6)** | −0.6 (3.6)* | −1.4 (3.6) |
| LOCF population | |||
| n | 193 | 186 | 198 |
| Baseline score | 18.1 (4.5) | 18.7 (4.6) | 18.8 (4.6) |
| Week 26 change | 0.4 (3.4)** | −0.4 (3.5)* | −1.4 (3.5) |
ADAS‐cog, cognitive subscale of the Alzheimer's Disease Assessment Scale; BID, twice daily; CIBIC‐Plus, Clinician Interview Based Impression of Change incorporating caregiver information; GDS, Global Deterioration Scale; ITT, intention to treat; LOCF, last observation carried forward; MMSE, Mini‐Mental State Examination; OC, observed cases; PDS, Progressive Deterioration Scale; TID, three times daily.
CIBIC‐Plus p values based on pairwise t tests using pooled error term from ANOVA (SAS Type III); ADAS‐cog, ADAS‐cogA and PDS p values based on the Mantel–Haenszel test blocking for centre; MMSE and GDS p values based on pairwise t tests using pooled error term from ANCOVA/ANOVA (SAS Type III analysis).
Values are mean (SD).
*p<0.05; **p⩽0.001.
Figure 3 Responder analysis of the cognitive subscale of the Alzheimer's Disease Assessment Scale: patient improvement in the intention to treat population administered rivastigmine twice (BID) or three time (TID) daily, or placebo (PBO). *p<0.05, **p⩽ 0.001 vs placebo. p Values based on pairwise Mantel–Haenszel tests.
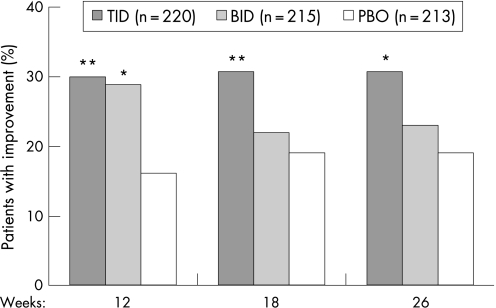
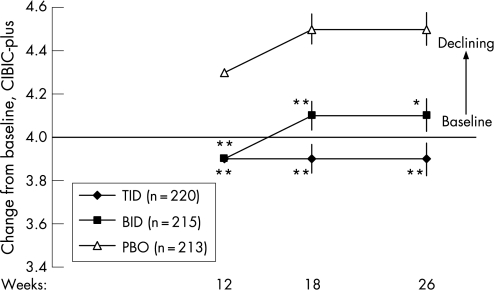
Significant differences in the proportions of patients showing improvements on the CIBIC‐Plus were observed between the rivastigmine TID and placebo groups at weeks 12, 18 and 26. Differences between the rivastigmine BID and placebo groups were significant at 12 weeks (fig 4). Changes from baseline on the CIBIC‐Plus in the ITT population are shown in fig 5. Similar results were obtained with the LOCF and OC analyses (data not shown).
Figure 4 Categorical analysis of the Clinician Interview Based Impression of Change incorporating caregiver information: patient improvement in the intention to treat population administered rivastigmine twice (BID) or three time (TID) daily, or placebo (PBO). *p<0.05, **p = 0.001 vs placebo. p Values based on pairwise Mantel–Haenszel tests.
Figure 5 Changes from baseline on the Clinician Interview Based Impression of Change incorporating caregiver information (CIBIC‐Plus) in the intention to treat population administered rivastigmine twice (BID) or three time (TID) daily, or placebo (PBO). *p<0.05, **p⩽0.001 vs placebo. p Values based on pairwise t tests using pooled error term from ANCOVA/ANOVA (SAS Type III). Values are mean (SEM).
On secondary outcome measures, the mean ADAS‐cogA scores at the endpoint showed significant treatment differences in both rivastigmine groups versus placebo. There was significantly less deterioration in activities of daily living on the PDS in both rivastigmine groups than in the placebo group (table 3). These findings were supported by LOCF analyses.
Cohen's D effect sizes for the ADAS‐cog and the CIBIC‐Plus are presented in table 4.
Table 4 Cohen's D effect sizes for the ADAS‐cog and CIBIC‐Plus.
| Week | Rivastigmine TID vs placebo | Rivastigmine BID vs placebo |
|---|---|---|
| D | D | |
| ADAS‐cog | ||
| 12 | 0.48 | 0.30 |
| 18 | 0.52 | 0.30 |
| 26 | 0.41 | 0.22 |
| CIBIC‐Plus | ||
| 12 | 0.40 | 0.40 |
| 18 | 0.54 | 0.36 |
| 26 | 0.46 | 0.31 |
ADAS‐cog, cognitive subscale of the Alzheimer's Disease Assessment Scale; BID, twice daily; CIBIC‐Plus, Clinician Interview Based Impression of Change incorporating caregiver information; TID, three times daily.
Safety and tolerability
Table 5 presents the adverse events (AEs) experienced within this RCT. Overall, dizziness was reported more frequently in both rivastigmine groups than in the placebo group (TID vs placebo p<0.01; BID vs placebo p<0.01) while headache (BID vs placebo p = 0.03) and anxiety (BID vs placebo p = 0.02) were reported more frequently in the rivastigmine BID group. Agitation (placebo vs TID p = 0.05) and haemorrhoids (placebo vs TID p = 0.17) were reported more frequently in the placebo than in the rivastigmine TID group.
Table 5 Adverse events occurring at significantly different frequencies in the rivastigmine TID or BID groups relative to placebo.
| Adverse event | Rivastigmine TID (n = 227) | Rivastigmine BID (n = 228) | Placebo (n = 222) |
|---|---|---|---|
| Any adverse event | 91.6* | 91.2* | 76.1 |
| Serious adverse events | 17.6 | 17.5 | 14.9 |
| Discontinuations for any adverse event | 10.6 | 16.7* | 9.0 |
| Nausea | 48.0* (4.4*) | 53.9* (4.8*) | 14.0 (0.5) |
| Vomiting | 30.0* (1.3) | 38.6* (4.4*) | 6.3 (0.5) |
| Diarrhoea | 16.7* (0.4) | 17.5* (0.9) | 9.0 (0.9) |
| Anorexia | 18.5* (2.2) | 20.6* (1.8) | 2.7 (0.0) |
| Abdominal pain | 11.5* (0.4) | 14.9* (1.8) | 5.4 (0.0) |
| Flatulence | 6.6* (0.0) | 4.8 (0.4) | 1.8 (0.0) |
| Headache | 15.9 (0.4) | 17.5* (0.9) | 10.4 (0.0) |
| Dizziness | 17.2* (0.9) | 18.4* (0.0) | 7.2 (0.0) |
| Agitation | 6.2* (0.0*) | 9.2 (1.3) | 11.7 (2.3) |
| Anxiety | 3.5 (0.0) | 5.7* (0.4) | 1.4 (0.0) |
| Haemorrhoids | 0.9 (0.0) | 0.0* (0.0) | 2.7 (0.0) |
BID, twice daily; TID, three times daily.
Values are shown as percentages of patients reporting the events, with percentages withdrawing from the study in parentheses (safety population).
*p<0.05 vs placebo, based on Fisher's exact test.
AEs were significantly more commonly reported in both the titration and maintenance phases in the rivastigmine groups than in the placebo group (p<0.05) but these AEs most often did not lead to withdrawal from the study. The most significantly more commonly reported AEs with rivastigmine compared with placebo included nausea, vomiting, anorexia and abdominal pain. In the rivastigmine groups, more patients experienced nausea during the titration phase (43% TID, 50% BID, 10% placebo) than during the maintenance phase (18% TID, 24% BID, 5% placebo).
A similar proportion of patients in each treatment group experienced at least one serious adverse event (any event that was fatal, considered life threatening or required hospitalisation): 18% in the rivastigmine TID group, 18% in the BID group and 15% in the placebo group. The two main causes for a serious adverse event in all groups were recorded as “overdose” (4–6%) and “unspecified procedural complications” (1–2%), with no other single type of event comprising >1% of the total incidence, and no significant difference in incidence of any individual type of event between the treatment groups (all NS vs placebo). Most of these events were defined as “serious” because they required hospitalisation (14% in the TID group, 13% in the BID group and 12% in the placebo group). Withdrawal because of adverse events accounted for 17% of BID, 11% of TID and 9% of placebo patients. In the TID group, approximately 33% of patients were receiving less than 6 mg/day at the time of withdrawal and approximately 66% were taking at least 6 mg/day, whereas in the BID group closer to 50% were receiving less than or at least 6 mg/day, so the greater discontinuation rate in the BID group was not a reflection of higher doses taken in this group (the exact factors contributing to the higher discontinuation rate are not known). No patients died during the study period.
Rivastigmine produced no clinically relevant changes in laboratory tests, physical examination findings or in vital signs. There was a small but statistically significant decrease in mean weight with rivastigmine treatment (mean change −1.27 (SD 3.57) kg in the TID group and −1.56 (3.92) kg in the BID group; both p<0.001) compared with placebo, where there was a mean increase at week 26 (+0.71 (3.28) kg). At 26 weeks, heart rate decreased from baseline by 2.9 beats per minute in the TID group (p = 0.02 vs placebo), 2.5 beats per minute in the BID group (p = 0.04) and 0.2 beats per minute in the placebo group. There were two cases of bradycardia reported as AEs during the study: one in the placebo group and one in the TID group. Overall, there were no clinically meaningful differences between treatment groups in quantitative ECG parameters during the study. Mean changes from baseline in PR intervals were 2.6 ms in the TID group, −0.3 ms in the BID group and −0.8 ms in the placebo group (p<0.05 for TID vs placebo); mean changes in QRS durations were 0.7, 0.8 and 0.0 ms, respectively (both NS); mean changes from baseline in QT intervals were 2.7, 5.3 and 2.4 ms (both NS); and changes in corrected QT intervals were −5.3, −1.7 and 1.4 ms (p<0.05 for TID vs placebo).
Discussion
This placebo controlled RCT demonstrated that rivastigmine administered both as a BID and TID regimen significantly benefited cognition, the ability to perform activities of daily living and global function in mild to moderately severe AD. The spectrum of the most frequent adverse events was similar to previous RCTs that have been reported with rivastigmine, with gastrointestinal cholinergic side effects being most common. Although nausea and vomiting each exceeded 30% adverse event rates, the rates of discontinuation related to these adverse events were far lower (4% and 5%, respectively), supporting the clinical experience that these adverse events are most often transient and not often associated with the need to discontinue medication.
This study utilised a forced dose titration schedule to the highest tolerated dose within the first 12 weeks of treatment, with weekly dose increments of 1 mg. This contrasts with usual clinical practice where clinicians aim to increase the daily dose by 3 mg monthly to achieve the highest tolerated daily dose in the range of 6–12 mg. Using this approach there were 71% who reached 9–12 mg in the TID group and 60% in the BID group. The completer rate was higher and the maximally tolerated mean dose of rivastigmine was higher in the TID arm. In fact, the completer rate of the TID treatment arm in this RCT is noted to be among the highest reported to date for rivastigmine,9,10,22,23,24,25,26 with drop‐out rates related to adverse events that were very close to placebo rates.
This study was not prospectively designed to evaluate whether TID administration has superior efficacy and safety to BID dosing. Rather, the study was designed to test each regimen against placebo. There were differences seen in the efficacy and tolerability between the TID and BID regimens however, and these differences must be interpreted with considerable caution as they were found through post hoc exploratory analyses, which are hypothesis generating. On the efficacy side, the ADAS‐cog mean change and responder analyses showed significant treatment effects in both the TID and BID groups at all time points. On the CIBIC‐Plus, the mean change in treatment effect was significant across all time points but the responder analysis was significant only at week 12 in the BID group. The reasons for this discrepancy are unknown but this underscores that this instrument may yield different results using different analytical techniques.
There is no consensus as to whether it is the responder analysis or mean change on the CIBIC‐Plus that is more clinically relevant. The Cohen's D effect sizes were included in our post hoc exploratory analyses because such calculations of effect sizes make possible between study as well as within study comparisons. Cohen's D is one of the most widely used measures of magnitude of effect whereby values approximating 0.2 suggest “small” effects, 0.5 suggest “medium” effects and 0.8 “large” effects.27 The Cohen's D analyses were supportive of the finding that primary effect sizes for the TID dose were larger on both the cognitive and global outcomes than the BID dose arm—according to Cohen's D, effect sizes seen in the TID group were “medium” sized whereas those in the BID group were “small”.
From the safety and tolerability standpoint, the rates of the most common AEs were very similar between the TID and BID groups. There were, however, significantly more discontinuations for AEs in the BID compared with the placebo group whereas this was not the case for the TID–placebo comparison. The discontinuation rate of the TID group was very similar to the placebo drop‐put rate while the proportion of TID subjects able to reach the maximal dose level of 12 mg/day was higher than in the BID group. Compared with the other 24 week RCTs of rivastigmine, which reported discontinuation rates in the range of 35% in patients receiving rivastigmine 6–12 mg/day BID,9,10 the TID group in the current study showed the lowest withdrawal rates reported. These findings may indicate that there is some aspect of tolerability that is better using the medication on a TID basis. We would speculate that this could be related to lower peak concentrations (Cmax) of rivastigmine in the brain. It has been previously reported that AEs observed with ChE‐Is appear to be caused by rapid, maximal increases in brain levels of ACh.11,12,28 Following oral administration, plasma and brain concentrations of rivastigmine quickly increase to a peak as the drug is rapidly absorbed from the gastrointestinal tract and readily crosses the blood–brain barrier.8 When titration is performed too rapidly, an increased incidence of centrally mediated cholinergic side effects such as nausea and vomiting occur.11 Through more frequent administration of smaller doses of rivastigmine over a 24 h period, the TID regimen may have afforded exposures associated with therapeutic effects at lower peak concentrations and improved tolerability. It has been previously noted that administration of rivastigmine with or shortly after food can also improve its tolerability as this not only slows the rate of increase but also reduces peak plasma concentrations of the drug.
Extending from the current data, it might be speculated that alternative formulations such as transdermal patches that avoid the significant first pass effect seen with oral administration, and which achieve slower increases to lower peak plasma concentrations with more even exposure over a 24 h period, could offer distinct advantages over the short lived plasma peaks of rivastigmine induced by its current capsule formulations. This might afford improved treatment compliance, initiation at higher doses with fewer side effects and a simpler titration regimen without the need for concurrent food intake.
The majority of adverse events, and discontinuations, in this study occurred during the rapid, forced dose titration phase, an approach that is discordant with usual clinical practice and which may limit its generalisability. In clinical settings, it is rarely necessary to attain maximum doses on a rapid forced titration basis.
The regulatory labelling for the class of ChE‐Is in AD warns that these compounds can slow heart rate and may induce syncope. Because of their pharmacological actions, ChE‐Is have the potential for vagotonic effects that may lead to bradycardia (heart rate lower than 50 beats per minute for >30 s). In the current study, small but statistically significant changes in heart rate, PR and corrected QT intervals were reported in rivastigmine treated patients. These electrocardiographic changes did not appear to be associated with a clinically increased (or significant) rate of cardiac AEs in the rivastigmine groups, compared with placebo, in the current study. Rivastigmine has previously been associated with a favourable cardiac safety profile in patients with AD, dementia with Lewy bodies or Parkinson's disease dementia.29,30
The current data provide important evidence that TID dosing of rivastigmine could be a therapeutic option for patients who face tolerability problems to rivastigmine treatment, particularly in circumstances where a BID titration approach is not advancing or to achieve a well tolerated higher daily dose.
Acknowledgements
Data collection and comments on study design were made by the International Exelon Investigators. HF was the principal investigator for the study, the principal writer and participated in designing and executing the study. RL contributed to analysing data and wrote the first draft of the paper.
The authors express their appreciation to Dr Claudia Jacova, Division of Neurology, University of British Columbia, for having assisted in the Cohen D effect size analyses.
Abbreviations
ACh - acetylcholine
AChE - acetylcholinesterase
AD - Alzheimer's Disease
ADAS‐cog - cognitive subscale of the Alzheimer's Disease Assessment Scale
AE - adverse event
BID - twice daily
BuChE - butyrylcholinesterase
ChE‐Is - cholinesterase inhibitors
CIBIC‐Plus - Clinician Interview Based Impression of Change incorporating caregiver information
GDS - Global Deterioration Scale
ITT - intention to treat
LOCF - last observation carried forward
MMSE - Mini‐Mental State Examination
OC - observed cases
PDS - Progressive Deterioration Scale
RCT - randomised controlled trial
TID - three times daily
Appendix
Australia: T Broe, J Hecker, R Helme, M Leong; Canada: R Eastwood, K Farcnik, H Feldman, E Mohr; Italy: G Abate, L Bartorelli, C Carapezzi, A Defanti, G Gainotti, F Girotti, G Nappi, P Nichelli, C Pettenati, A Ranzenigo, U Senin, H Spinnler, G Valenti; South Africa: C Gagiano, M Page, F Potocnik; UK: D Bates, A Bayer, CJ Bulpitt, L Findley, JR Hodges, J Kellet, R Lewis, J Lindesay, M McMurdo, IK Mutiboko, A O'Brien, S Olivleri, A Phanjoo, M Rossor, LJ Whalley.
Footnotes
This study was supported by funding from Novartis Pharma AG, Basle, Switzerland.
Competing interests: HF has received honoraria for consulting, advisory boards and for participation in CME programs sponsored by Novartis. He has also received grant‐in‐aid funding for research from Novartis. RL is an employee of Novartis. The study was commissioned by Novartis Pharma AG in Switzerland.
References
- 1.Davies P, Maloney A J. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet 197621403. [DOI] [PubMed] [Google Scholar]
- 2.Mesulam M M, Guillozet A, Shaw P.et al Widely spread butyrylcholinesterase can hydrolyse acetylcholine in the normal and Alzheimer brain. Neurobiol Dis 2002983–93. [DOI] [PubMed] [Google Scholar]
- 3.Doody R S, Stevens J C, Beck C.et al Practice parameter: management of dementia (an evidence‐based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001561154–1166. [DOI] [PubMed] [Google Scholar]
- 4.Farlow M. A clinical overview of cholinesterase inhibitors in Alzheimer's disease. Int Psychogeriatr 200214(Suppl 1)93–126. [DOI] [PubMed] [Google Scholar]
- 5.Brufani M, Filocamo L. Rational design of cholinesterase inhibitors. In: Giacobini E, ed. Cholinesterase and cholinesterase inhibitors. London: Martin Dunitz, 200027–46.
- 6.Darreh‐Shori T, Almkvist O, Guan Z Z.et al Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology 200259563–572. [DOI] [PubMed] [Google Scholar]
- 7.Cutler N R, Polinsky R J, Sramek J J.et al Dose‐dependent CSF acetylcholinesterase inhibition by SDZ ENA 713 in Alzheimer's disease. Acta Neurol Scand 199897244–250. [DOI] [PubMed] [Google Scholar]
- 8.Polinsky R J. Clinical pharmacology of rivastigmine: a new‐generation acetylcholinesterase inhibitor for the treatment of Alzheimer's disease. Clin Ther 199820634–647. [DOI] [PubMed] [Google Scholar]
- 9.Corey‐Bloom J, Anand R, Veach J. for the ENA 713 B352 Study Group. A randomized trial evaluating efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer's disease. Int J Geriatr Psychopharmacol 1998155–65. [Google Scholar]
- 10.Rösler M, Anand R, Cicin‐Sain A.et al Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomised controlled trial. BMJ 1999318633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossberg G T. Cholinesterase inhibitors for the treatment of Alzheimer's disease: Getting on and staying on. Curr Ther Res 200364216–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jhee S S, Shiovitz T, Hartman R D.et al Centrally acting antiemetics mitigate nausea and vomiting in patients with Alzheimer's disease who receive rivastigmine. Clin Neuropharmacol 200225122–123. [DOI] [PubMed] [Google Scholar]
- 13.Sikdar S. Should titration schedules for cholinesterase inhibitors be changed? Int J Geriatr Psychiatry 2003181063–1064. [DOI] [PubMed] [Google Scholar]
- 14.Forette F, Anand R, Garabawi G. A phase II study in patients with Alzheimer's disease to assess the preliminary efficacy and maximum tolerated doses of rivastigmine (Exelon). Eur J Neurol 19996423–429. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association ( A P A )Diagnostic and statistical manual of mental disorders. Washington DC: APA, 1994
- 16.McKhann G, Drachman D, Folstein M.et al Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 198434939–944. [DOI] [PubMed] [Google Scholar]
- 17.Folstein M F, Folstein S E, McHugh P R. ‘Mini‐Mental State': a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 197512189–198. [DOI] [PubMed] [Google Scholar]
- 18.Rosen W G, Mohs R C, Davis K L. A new rating scale for Alzheimer's disease. Am J Psychiatry 19841411356–1364. [DOI] [PubMed] [Google Scholar]
- 19.Reisberg B, Schneider L, Doody R.et al Clinical global measures of dementia. Position paper from the International Working Group on Harmonization of Dementia Drug Guidelines. Alzheimer Dis Assoc Disord 199711(Suppl 3)8–18. [PubMed] [Google Scholar]
- 20.DeJong R, Osterlund O W, Roy G W. Measurement of quality‐of‐life changes in patients with Alzheimer's disease. Clin Ther 198911545–554. [PubMed] [Google Scholar]
- 21.Reisberg B, Ferris S H, de Leon M J.et al The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry 19821391136–1139. [DOI] [PubMed] [Google Scholar]
- 22.Agid Y, Dubois B on behalf of the International Rivastigmine Investigators, Anand R, Gharabawi G Efficacy and tolerability in patients with dementia of the Alzheimer type. Curr Ther Res 199859837–846. [Google Scholar]
- 23.Anand R, Gharabawi G, Enz A. Efficacy and safety results of the early phase studies with Exelon (ENA‐713) in Alzheimer's disease: an overview. J Drug Dev Clin Pract 19968109–116. [Google Scholar]
- 24.Bullock R, Touchon J, Bergman H.et al Rivastigmine and donepezil treatment in moderate to moderately‐severe Alzheimer's disease over a 2‐year period. Curr Med Res Opin 2005211317–1327. [DOI] [PubMed] [Google Scholar]
- 25.Emre M, Aarsland D, Albanese A.et al Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med 20043512509–2518. [DOI] [PubMed] [Google Scholar]
- 26.McKeith I G, Wesnes K A, Perry E.et al Hallucinations predict attentional improvements with rivastigmine in dementia with lewy bodies. Dement Geriatr Cogn Disord 20041894–100. [DOI] [PubMed] [Google Scholar]
- 27.Cohen J.Statistical power analysis for the behavioural sciences, 2nd edn. Hillsdale, NJ: Erlbaum 1988
- 28.Darvesh S, Walsh R, Kumar R.et al Inhibition of human cholinesterases by drugs used to treat Alzheimer's disease. Alzheimer Dis Assoc Disord 200317117–126. [DOI] [PubMed] [Google Scholar]
- 29.Morganroth J, Graham S, Hartman R.et al Electrocardiographic effects of rivastigmine. J Clin Pharmacol 200242558–568. [DOI] [PubMed] [Google Scholar]
- 30.Ballard C, Lane R, Barone P.et al Cardiac safety of rivastigmine in Lewy body and Parkinson's disease dementias. Int J Clin Pract 200660639–645. [DOI] [PubMed] [Google Scholar]