Abstract
Background
123I‐labelled 2β‐carbomethoxy‐3β‐(4‐iodophenyl)‐N‐(3‐fluoropropyl) nortropane (123I‐FP‐CIT) imaging is a diagnostic tool to help differentiate dementia with Lewy bodies (DLB) from Alzheimer's disease (AD). However, in animals, cholinesterase inhibitors (ChEi) have been reported to reduce radioligand binding to the striatal dopamine transporter. As ChEi are frequently used in people with dementia, it is important to determine whether their use affects 123I‐FP‐CIT uptake in the striatum.
Objective
To clarify whether chronic ChEi therapy modulates striatal dopamine transporter binding measured by 123I‐FP‐CIT in patients with AD, DLB and Parkinson's disease with dementia (PDD).
Design
Cross sectional study in 99 patients with AD (nine on ChEi, 25 not on ChEi), DLB (nine on ChEi, 19 not on ChEi) and PDD (six on ChEi, 31 not on ChEi) comparing 123I‐FP‐CIT striatal binding (caudate, anterior and posterior putamen) in patients receiving compared with those not receiving ChEi, correcting for key clinical variables including diagnosis, age, sex, Mini‐Mental State Examination score, severity of parkinsonism and concurrent antidepressant use.
Results
As previously described, 123I‐FP‐CIT striatal uptake was lower in DLB and PDD subjects compared with those with AD. Median duration of ChEi use was 180 days. 123I‐FP‐CIT uptake was not significantly reduced in subjects receiving ChEi compared those not receiving ChEi (mean percentage reduction: AD 4.3%; DLB 0.7%; PDD 6.1%; p = 0.40). ChEi use did not differentially affect striatal 123FP‐CIT uptake between patient groups (p = 0.83).
Conclusions
Use of ChEi does not significantly influence the ability of 123I‐FP‐CIT imaging to distinguish AD from DLB.
Single photon emission computed tomography (SPECT) with the ligand 123I‐labelled 2β‐carbomethoxy‐3β‐(4‐iodophenyl)‐N‐(3‐fluoropropyl) nortropane (123I‐FP‐CIT), which binds to the dopamine transporter (DAT), has been shown to be useful in discriminating dementia with Lewy bodies (DLB) from Alzheimer's dementia (AD).1,2
However, the utility of 123I‐FP‐CIT imaging may be undermined if individuals are taking medications that affect the striatal binding of 123I‐FP‐CIT, particularly if such agents differentially influence 123I‐FP‐CIT striatal binding between DLB and AD. A significant class of drugs which might impact on 123I‐FP‐CIT binding in these patient groups are cholinesterase inhibitors (ChEi). In the monkey, DAT availability, assessed by [11C] β‐CFT positron emission tomography (PET), was reported to be acutely suppressed in a dose dependent manner by the ChEi donepezil.3 Direct evidence that cholinesterase inhibition modulates DAT function in humans is currently lacking.
To determine whether chronic ChEi therapy influences striatal DAT binding by 123I‐FP‐CIT in humans, we compared 123I‐FP‐CIT striatal binding in patients with AD, DLB and Parkinson's disease with dementia (PDD) treated with ChEi with those not receiving these agents.
Methods
Data from 93 subjects from a previous study published by our group were analysed.2 Data from a further six subjects (five with DLB and one with PDD) were also included.
Detailed SPECT methodology and analysis are described elsewhere.2 In brief, striatal binding was determined from the striatal:occipital activity ratios. Regions of interest (ROIs) were positioned at three distinct locations within the striatum to obtain measurements of the caudate, anterior putamen and posterior putamen. Striatal:occipital activity ratios for the three ROIs for each hemisphere were determined as:
123I‐FP‐CIT bindingcaudate, anterior putamen, posterior putamen = Striatal uptakecaudate, anterior putamen, posterior putamen / Occipital uptake
For analysis, an average of left and right hemisphere 123I‐FP‐CIT uptake for each ROI was used.
Analysis of the effect of medications on 123I‐FP‐CIT striatal binding was tested using multivariate analysis of covariance (MANCOVA) with fixed factors of sex, diagnosis and concurrent antidepressant use, and covariates of age, Mini‐Mental State Examination score, Unified Parkinson's Disease Rating Scale, subsection III (UPDRS III) score and duration of illness. In subjects on medications, time on ChEi was added into the model as an additional covariate.
Results
Subject characteristics are summarised in table 1. Groups were broadly matched for sex, age and cognitive impairment although the PDD group were younger than the AD (p<0.001) and DLB (p<0.02) groups. As expected, the DLB and PDD groups had higher UPDRS III scores than the AD subjects (DLB vs AD, p<0.001; PDD vs AD, p<0.001). Analysis of illness duration showed that PDD subjects had a longer illness duration compared with AD (p<0.001) and DLB (p<0.001) subjects. Subject demographics for age, sex, degree of cognitive impairment or UPDRS III score did not differ by use of ChEi. Subjects with PDD taking ChEi had a longer illness duration (p = 0.05) and a trend to have been on their medication longer than subjects in the AD and DLB groups (p = 0.07). Concurrent antidepressant use was similar between diagnostic groups (p = 0.47) and cholinesterase status (p = 0.70).
Table 1 Demographic and neuropsychological data on subjects studied using 123I‐FP‐CIT SPECT.
| Variable | Subjects | p Value (diagnosis group) | p Value (ChEi status) | |||||
|---|---|---|---|---|---|---|---|---|
| With AD | With DLB | With PDD | ||||||
| No ChEi (n = 25) | ChEi (n = 9) | No ChEi (n = 19) | ChEi (n = 9) | No ChEi (n = 31) | ChEi (n = 6) | |||
| Sex (M/F) | 10/15 | 5/4 | 13/6 | 4/5 | 20/11 | 6/0 | 0.08 | 0.66 |
| Age (y) | 79.1 (5.7) | 78.5 (5.6) | 74.3 (6.6) | 79.4 (6.7) | 72.3 (5.3) | 70.3 (6.3) | <0.001† | 0.56 |
| MMSE score (maximum score 30) | 17.9 (5.1) | 15.6 (4.0) | 15.6 (6.1) | 18.0 (4.3) | 19.0 (5.2) | 20.2 (7.9) | 0.15 | 0.72 |
| CAMCOG (maximum score 107) | 59.8 (15.9) | 49.3 (21.7) | 59.6 (16.4) | 61.5 (8.2) | 63.8 (14.8) | 64.3 (16.4) | 0.09 | 0.64 |
| UPDRS III (maximum score 108) | 4.9 (3.9) | 7.4 (7.0) | 27.8 (14.1) | 20.1 (9.8) | 37.3 (11.6) | 36.7 (11.8) | <0.001‡ | 0.44 |
| Duration of Illness (months) | 33.2 (20.1) | 38.3 (13.5) | 33.6 (25.9) | 27.6 (25.0) | 65.1 (39.0) | 133 (96.0) | <0.001¶ | 0.01§ |
| Time on ChEi prior to 123I‐FP‐CIT (days)* | NA | 99 (26–733) | NA | 124 (35–520) | NA | 388 (128–577) | 0.07 | NA |
| Antidepressant use (n) | 2 | 3 | 3 | 1 | 8 | 1 | 0.47 | 0.70 |
AD, Alzheimer's disease; CAMCOG, Cambridge Cognitive Examination; ChEi, acetylcholinesterase inhibitor; DLB, dementia with Lewy bodies; 123I‐FP‐CIT, 123I‐labelled 2β‐carbomethoxy‐3β‐(4‐iodophenyl)‐N‐(3‐fluoropropyl) nortropane; MMSE, Mini‐Mental State Examination; NA, not applicable; PDD, Parkinson's disease with Lewy body dementia; UPDRS III, Unified Parkinson's Disease Rating Scale subsection III: motor function.
Data are mean (SD) unless stated otherwise.
*Data expressed as median (minimum to maximum).
†Gabriel post hoc tests showed that subjects with AD were older than PDD subjects (p<0.001) and subjects with DLB were older than PDD subjects (p<0.02).
‡Mann–Whitney post hoc tests showed that subjects with AD had lower UPDRS III scores than those with DLB (p<0.001) or PDD (p<0.001). Subjects with DLB had lower UPDRS III scores than those with PDD (p<0.01).
¶Mann‐Whitney post hoc tests showed that subjects with PDD had longer illness duration than AD (p<0.001) or DLB (p<0.001).
§Interaction between diagnosis group and ChEi status was significant (p<0.01). Mann‐Whitney post hoc tests showed that subjects with PDD and on ChEi had longer illness duration than PDD subjects not on ChEi (p = 0.05).
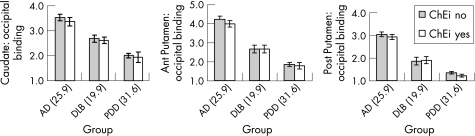
Figure 1 shows graphically the mean and 95% CI for the striatal:occipital binding ratios of 123I‐FP‐CIT for the three ROIs in subjects receiving ChEi and subjects not receiving ChEi. Striatal uptake differed between the groups (MANCOVA, Wilks Lambda, df = 6, F = 3.87, p = 0.001), after adjusting for fixed factors and covariates; specifically, the PDD group had lower striatal uptake in all three areas compared with the DLB (p<0.001) and AD (p<0.001) groups. DLB subjects had significantly lower uptake in all three striatal regions compared with the AD subjects (p<0.001). With regard to the effects of ChEi, across all three ROIs, there was a tendency for uptake to be reduced in those subjects on ChEi (mean magnitude of striatal:occipital binding reduction: AD 4.3%; DLB 0.7%; PDD 6.1%) but this reduction was not significant in the corrected multivariate analysis (MANCOVA, Wilks Lambda, df = 3, F = 0.99, p = 0.40). There was no significant interaction between the use of ChEi and diagnostic group in terms of striatal 123I‐FP‐CIT uptake (MANCOVA, Wilks Lambda, df = 3, F = 0.47, p = 0.83). Similarly, antidepressant use did not significantly affect 123I‐FP‐CIT uptake (MANCOVA, Wilks Lambda, df = 3, F = 0.28, p = 0.84) and there was no significant interaction between ChEi and concurrent antidepressant use (MANCOVA, Wilks Lambda, df = 3, F = 0.26, p = 0.96). In subjects taking ChEi, duration of prior ChEi use did not impact significantly on 123I‐FP‐CIT uptake (MANCOVA, Wilks Lambda, df = 3, F = 0.60, p = 0.54).
Figure 1 Striatal:occipital activity for subjects with Alzheimer's disease (AD), dementia with Lewy bodies (DLB) and Parkinson's disease with dementia (PDD) in the caudate nucleus, anterior putamen and the posterior putamen comparing subjects receiving cholinesterase inhibitors (ChEi) and those not receiving ChEi. Values are means (95% CI).
Discussion
123I‐FP‐CIT SPECT imaging has been shown to have high sensitivity and specificity in distinguishing AD from DLB, even in DLB patients without clinical parkinsonism.1,2,4,5 The main finding of this study is that concurrent use of ChEi does not significantly affect the results from 123I‐FP‐CIT imaging.
There are conflicting reports on whether ChEi influence DAT binding by radioligands. In rats, Kilbourn et al showed that treatment with phenserine, a ChEi, produced a 24% decrease in radioligand DAT binding of d‐threo‐[3H]methylphenidate.6 In contrast, in a biodistribution study in rats, Knol et al did not observe any effect of donepezil or rivastigimine on DAT imaging with 123I‐FP‐CIT.7 Relevant to the present study is the primate study of Tsukada et al who observed that a single dose of donepezil suppressed DAT availability, assessed using [11C] β‐CFT PET.3 Tsukada et al proposed that this effect may occur either via transynaptic mechanisms or presynaptic receptor modulation. This observation is contrary to that observed in the present study. Dosage levels of ChEi in the study of Tsukada et al and the present study were comparable. Possible explanations for this discrepancy include the following.
Differences in ligands
123I‐FP‐CIT reaches striatal binding equilibrium 3–6 h after injection. In contrast, [11C]β‐CFT reaches peak equilibrium within 1 h after injection.8 Furthermore, 123I‐FP‐CIT has an affinity for the serotonin reuptake transporter.9 Therefore, it is feasible that differences in affinity and kinetic binding profiles between [11C] β‐CFT and 123I‐FP‐CIT ligand may account for the disparity between our results and those of Tsukada et al.3
Differences in species, age and pathological status
Human DAT differs morphologically and functionally from DAT in other species.10,11 Consequently, species differences in terms of ligand binding may be apposite. In addition, the monkeys used by Tsukada et al were young and healthy. Age related reductions in DAT have been noted in humans in both SPECT and PET studies and specifically in Lewy body disease, where there are significant losses of striatal DAT.4,5,12,13 Therefore, it is possible that suppression of DAT activity as a result of ChEi use may not have been apparent in the present study because of floor effects.
Differences in duration of exposure to ChEi
The monkeys studied by Tsukada et al were treated acutely with an intravenous bolus of donepezil.3 In contrast, in our study, patients had been receiving oral ChEi for a median of 180 days (minimum 26 days). It is plausible that prolonged exposure to ChEi might lead to a compensatory upregulation in DAT as a result of increased acetylcholine and therefore negation of any acute suppression. This is, in part, supported by evidence that the chronic administration of nicotinic agonists such as nicotine increase DAT mRNA expression in rats.14 Furthermore, Padilla et al observed in rats that repeated bolus doses of chlorpyrifos, a ChEi insecticide, caused an increase in DAT density in the striatum after 6 months, although they did not observe any changes in DAT density with chronic low dose exposure to chlorpyrifos.15
Increases in 123I‐β‐CIT striatal binding have been observed in subjects treated with the serotonin reuptake inhibitors, citalopram and paroxetine.16,17 This increase may be caused by an interaction between serotonin and dopamine systems in the striatum16 or increased 123I‐β‐CIT availability secondary to peripheral serotonin reuptake transporter blockade and decreased central serotonin reuptake transporter availability.17 Therefore, it could be argued that depression of striatal 123I‐FP‐CIT uptake by ChEi might potentially be masked by concurrent antidepressant use. However, the proportions of subjects taking antidepressants in the present study were similar in those taking (20.8%) and not taking (17.3%) ChEi. Furthermore, we did not observe any significant interaction between antidepressant use and concurrent ChEi use in terms of 123I‐FP‐CIT striatal binding.
The cross sectional nature of the present study cannot exclude subtle changes in 123I‐FP‐CIT striatal uptake as a result of chronic use of ChEi. In addition, while currently most 123I‐FP‐CIT imaging is carried out in patients with established dementia and on long term ChEi, the present study cannot determine the effect of acute/short term ChEi use. To answer these issues, a prospective blinded study comparing 123I‐FP‐CIT binding before and after ChEi administration would be required. However, our study has important implications as it means that the prior use of ChEi does not affect the diagnostic discriminant validity of 123I‐FP‐CIT imaging. This has clinical importance as it suggests that it is not necessary to withdraw ChEi prior to diagnostic 123I‐FP‐CIT imaging, an action which could cause deleterious effects on patient's cognitive function and behaviour.
Acknowledgements
This study was supported in part by the Medical Research Council. We thank GE Healthcare for supplying 123I‐FP‐CIT (DaTSCAN).
Abbreviations
AD - Alzheimer's disease
ChEi - cholinesterase inhibitors
DAT - dopamine transporter
DLB - dementia with Lewy bodies
123I‐FP‐CIT - 123I‐labelled 2β‐carbomethoxy‐3β‐(4‐iodophenyl)‐N‐(3‐fluoropropyl) nortropane
PDD - Parkinson's disease with dementia
PET - positron emission tomography
ROIs - regions of interest
SPECT - single photon emission computed tomography
UPDRS III - Unified Parkinson's Disease Rating Scale, subsection III
Footnotes
Competing interests: John O'Brien, Ian McKeith and Jim Patterson have acted as consultants for GE Healthcare. David Burn has received honoraria from Novartis and GE Healthcare.
References
- 1.Walker Z, Costa D C, Walker R W.et al Differentiation of dementia with Lewy bodies from Alzheimer's disease using a dopaminergic presynaptic ligand. J Neurol Neurosurg Psychiatry 200273134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Brien J T, Colloby S, Fenwick J.et al Dopamine transporter loss visualized with FP‐CIT SPECT in the differential diagnosis of dementia with Lewy bodies. Arch Neurol 200461919–925. [DOI] [PubMed] [Google Scholar]
- 3.Tsukada H, Nishiyama S, Ohba H.et al Cholinergic neuronal modulations affect striatal dopamine transporter activity: PET studies in the conscious monkey brain. Synapse 200142193–195. [DOI] [PubMed] [Google Scholar]
- 4.Piggott M A, Marshall E F, Thomas N.et al Striatal dopaminergic markers in dementia with Lewy bodies, Alzheimer's and Parkinson's diseases: rostrocaudal distribution. Brain 19991221449–1468. [DOI] [PubMed] [Google Scholar]
- 5.Villares J C, Stavale J N. Age‐related changes in the N‐methyl‐aspartate receptor binding sites within the human basal ganglia. Exp Neurol 2001171391–404. [DOI] [PubMed] [Google Scholar]
- 6.Kilbourn M R, Kemmerer E S, Desmond T J.et al Differential effects of scopolamine on in vivo binding of dopamine transporter and vesicular monoamine transporter radioligands in rat brain. Exp Neurol 2004188387–390. [DOI] [PubMed] [Google Scholar]
- 7.Knol R J J, de Bruin C M, Booij J. Is the [123I]FP‐CIT binding to striatal dopamine transporters influenced by acetylcholinesterase inhibitors? Eur J Nucl Med Mol Imaging 200633S211. [DOI] [PubMed] [Google Scholar]
- 8.Farde L, Ginovart N, Halldin C.et al A PET study of [11C][beta]‐CIT‐FE binding to the dopamine transporter in the monkey and human brain. Int J Neuropsychopharmacol 20003203–214. [DOI] [PubMed] [Google Scholar]
- 9.Laruelle M, Baldwin R M, Malison R T.et al SPECT imaging of dopamine and serotonin transporters with [123I]beta‐CIT: pharmacological characterization of brain uptake in nonhuman primates. Synapse 199313295–309. [DOI] [PubMed] [Google Scholar]
- 10.Patel A, Uhl G, Kuhar M J. Species differences in dopamine transporters: postmortem changes and glycosylation differences. J Neurochem 199361496–500. [DOI] [PubMed] [Google Scholar]
- 11.Lee S H, Rhee J, Koh J K.et al Species differences in functions of dopamine transporter: paucity of MPP+ uptake and cocaine binding in bovine dopamine transporter. Neurosci Lett 1996214199–201. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki M, Desmond T J, Albin R L.et al Striatal monoaminergic terminals in Lewy body and Alzheimer's dementias. Ann Neurol 200251767–771. [DOI] [PubMed] [Google Scholar]
- 13.Erixon‐Lindroth N, Farde L, Robins Wahlin T ‐ B.et al The role of the striatal dopamine transporter in cognitive aging. Psychiatry Res Neuroimaging 20051381–12. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Kim K Y, Kim J H.et al Chronic nicotine and smoking treatment increases dopamine transporter mRNA expression in rat midbrain. Neurosci Lett 200436329–32. [DOI] [PubMed] [Google Scholar]
- 15.Padilla S, Marshall R S, Hunter D L.et al Neurochemical effects of chronic dietary and repeated high‐level acute exposure to chlorpyrifos in rats. Toxicol Sci 200588161–171. [DOI] [PubMed] [Google Scholar]
- 16.Kugaya A, Seneca N M, Snyder P J.et al Changes in human in vivo serotonin and dopamine transporter availabilities during chronic antidepressant administration. Neuropsychopharmacology 200328413–420. [DOI] [PubMed] [Google Scholar]
- 17.de Win M M, Habraken J B, Reneman L.et al Validation of [(123)I]beta‐CIT SPECT to assess serotonin transporters in vivo in humans: a double‐blind, placebo‐controlled, crossover study with the selective serotonin reuptake inhibitor citalopram. Neuropsychopharmacology 200530996–1005. [DOI] [PubMed] [Google Scholar]