Abstract
Background
Neurofibromatosis type 1 (NF1) is a single gene disorder associated with a high frequency of cognitive deficits and a complex cognitive phenotype. These cognitive deficits have been associated with focal areas of high signal intensity on T2 weighted MRI images but the relationship remains controversial.
Method
A cohort of 76 children with NF1 and 45 unaffected sibling controls (aged 8–16 years) underwent extensive neuropsychological assessment, with the NF1 children having MRI examinations.
Results
The presence or number of T2 hyperintensities (T2H) was not associated with cognitive dysfunction. However, the location of discrete (well circumscribed) T2H in the thalamus was associated with severe and generalised cognitive impairment. More diffuse lesions in the thalamus were also associated with reductions in IQ but the effects were less marked compared with the discrete lesions. Comparing children with NF1 to their unaffected siblings revealed more subtle effects of the lesions on cognitive ability.
Conclusions
T2H cannot be used in general as a radiological marker for cognitive deficits in children with NF1; however, lesions in the thalamus are strongly associated with cognitive impairment. It is possible that lesions in the thalamus in conjunction with more general thalamic hypometabolism may compound the level of thalamic dysfunction, resulting in cognitive deficits well beyond those produced by T2H in other regions.
Neurofibromatosis type 1 (NF1) is a neurocutaneous disorder with a prevalence of approximately 1 in 3500.1 One of the most common complications of NF1 in childhood is cognitive dysfunction. The most frequent problems reported are with attention, perception, executive functioning and academic achievement.2 NF1 is also associated with an increased risk for many CNS abnormalities, the most common being T2 hyperintensities (T2H), present in 60–70% of children with NF1.3 These lesions are usually isointense on T1 weighted images, exert no mass effect, do not enhance with contrast, are not associated with focal neurological deficits and tend to resolve by adulthood.4 They most commonly occur in the basal ganglia, brainstem, thalamus, optic tracts and cerebellum.
The potential relationship between these MRI T2H and cognitive dysfunction in NF1 remains controversial. While some studies have found no association between T2H and cognitive ability,5,6,7,8,9 several recent studies have found a relationship between cognitive dysfunction and the presence,10,11,12 number13 and location of T2H.14,15 The interpretation of results from many studies is hampered by small sample size, wide age range (ie, inclusion of adult patients in whom T2H may have resolved), variability in the definition of cognitive deficits and T2H, and the inclusion of patients with other intracranial pathology that may influence the presence of T2H and cognitive performance. On this basis we aimed to determine whether the presence, number and/or location of T2H are predictive of either general intellectual functioning or specific cognitive/learning deficits in a larger cohort of NF1 children (without any other significant cranial pathology).
Method
Subjects
All patients were recruited from the Neurogenetics Clinic at the Royal Alexandra Hospital for Children (Children's Hospital at Westmead) in Sydney, Australia. This study was conducted as part of a larger study examining the cognitive profile of children with NF1, where recruitment is described in detail.2 Exclusion criteria included CNS pathology or other medical conditions that affected test performance (eg, epilepsy/seizures, optic gliomas, brain tumours, hydrocephalus). Of the 81 NF1 patients in the larger study, 76 agreed to undergo a cranial MRI. Of these 76 NF1 patients, 45 unaffected sibling controls were also recruited and underwent neuropsychological assessment. The number of recruited patients was based on the maximum number of patients that we were able to ascertain from our clinic. This study received ethics approval from both the Children's Hospital at Westmead Ethics Committee and the US Army Medical Research and Materiel Command.
Procedure
Neuropsychological testing was conducted individually over two morning sessions, and an MRI was scheduled immediately following one of these sessions. Parents of children on stimulant medication for attention deficit–hyperactivity disorder (ADHD) were requested not to give their children the medication on the days of testing (n = 5). The MRI examinations were performed on a magnet operating at 1.5 T (Philips ACS‐NT; Philips, the Netherlands). The scans assessed via visual inspection on a light box MRI sequences were sagittal T1, axial T2, axial fluid attenuated inversion recovery (FLAIR), volume T2 (T2V), coronal FLAIR and axial T1 weighted imaging following intravenous administration of gadopentate dimeglumine at 0.1 mmol/kg (Magnevist). Typical imaging parameters were TR 500/TE 15 for axial T1 images pre and post contrast, TR 3600/TE 120 for single contrast axial T2 sequences and TR 7000/TE 130 IR delay 2200 ms for axial FLAIR. T2V parameters were TR 7000/TE 110. The images were obtained with 5 mm thick sections with 1 mm spacing, 220 cm field of view and a 512×256 matrix, except for T2V where 100 sections were obtained each 1.60 mm in thickness, giving an almost contiguous appearance.
The MRI examinations were reported independently by the radiologist on duty at the time of performance of the MRI, and then separately by an independent radiologist (AS) and a neurologist (DG). Two neurologists (DG and KN) reported the scans together, 1 year after the initial analysis. DG and AS were blinded to the clinical history other than NF1; all reporters were blinded to the results of other reporters. The results of independent reporting were then compiled to reach a consensus view. Each area of abnormal signal intensity on T2 weighted images was assigned as being either discrete or diffuse. Discrete lesions were those that were well circumscribed, having a margin that was distinct from normal tissue. Diffuse lesions were lesions that were not discrete; the margins of these lesions were poorly defined. There was complete agreement between all reporters for discrete lesions. There was occasional disagreement for diffuse lesions. In this case a consensus classification was reached by two investigators (DG and KN) and two radiologists (AS and SG) (methodology described in detail in Gill and colleagues16).
Measures
The following tests were administered: Wechsler Intelligence Scales for Children‐third edition,17 Wechsler Individual Achievement Test‐Screener (reading, spelling, mathematics),18 Test of Everyday Attention for Children‐Screener (selective, sustained, switching and divided attention),19 Conner's ADHD DSM‐IV Scales,20 Children's Category Test,21 Tower of London,22 Controlled Oral Word Association Test,23 Judgment of Line Orientation,24 Rey Complex Figure,25 Continuous Visual Memory Test,26 California Verbal Learning Test for Children,27 Grooved Pegboard28 and Test of Variables of Attention (to assess motor speed).29
Statistics
Three types of analyses were conducted. The effects of the presence of hyperintensities was analysed by independent t tests between those with T2H (T2H+) and those without T2H (T2H−), with the cognitive test scores as dependent variables. The frequency of comorbid disorders (ADHD and specific learning disability) between the two groups was examined using χ2 tests. The effect of the number of hyperintensities was examined using Spearman correlation coefficients between the number of T2H and cognitive test scores. The anatomical location of hyperintensities was analysed with t tests by comparing the scores of children with hyperintensities in a particular anatomical location with the scores of children both without hyperintensities or with hyperintensities elsewhere. The presence and location of T2H were examined with both discrete and total T2H count (ie, discrete plus diffuse lesions). For analysis of the number of lesions, only discrete lesions were used because of the difficulty in delineating the borders of the diffuse lesions.
Two different methods of calculating cognitive ability scores were performed. The first method, a simple comparison, used the standard scores on the cognitive tests to examine whether the presence, number or location of the T2H was predictive of their actual performance scores. The second technique used a pairwise comparison between the NF1 patient and their sibling controls (as performed in previous studies by Denckla and colleagues13,30). This method calculated a “difference score” by subtracting the NF1 patient's scores from their sibling controls. This score gives a reflection of the reduction in “potential familial” cognitive ability, taking into account both family environment and genetics. Both methods were conducted in the following analysis to determine whether T2 lesions are associated with either a general lowering of ability compared with the normal population or a specific lowering of ability compared with sibling controls.
Results
Patients with NF1 ranged in age from 8.0 to 16.75 years (mean 11.63 (SD 2.32) years). Among the patients, 47% were female and 53% were male, with 61% having sporadic NF1 and 39% familial NF1. Fifty per cent of the cohort had macrocephaly. Of the 76 patients, 54 (71.1%) had at least one discrete T2H whereas 22 (28.9%) had no evidence of any discrete lesions. When taking into account both discrete and diffuse lesions (total T2H), 68 patients (89.5%) had T2H and only eight patients (10.5%) had no lesions on T2 weighted images. Of the 54 patients who showed evidence of a discrete lesion, the number of lesions ranged from 1 to 7, with a mean of 2.96 (median 2, SD 1.78).
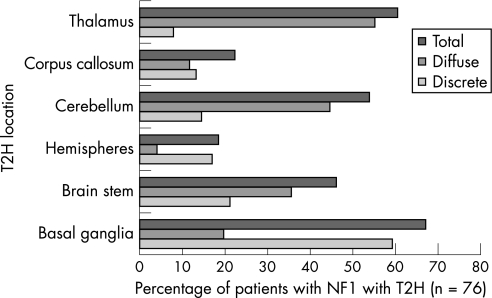
The location of T2H in the 76 NF1 children is shown in fig 1. It is noted that there are large differences between diffuse and discrete lesions in their location: basal ganglia and hemispheric T2H were more likely to be discrete whereas lesions in the thalamus, cerebellum and brainstem were more likely to be diffuse. When examining the 45 children with NF1 in whom sibling controls were also studied, there were no significant differences in the frequency, number or location of T2H between this subgroup of children with NF1 and the total NF1 group. Of the 45 pairs, 34 children with NF1 (75.6%) had discrete T2H and 41 (91.1%) has both discrete and diffuse T2H. The results of the cognitive assessment have been previously described in detail.2,31
Figure 1 Frequency of T2 hyperintensities (T2H) in different anatomical locations. NF1, neurofibromatosis type 1.
Relationship between MRI T2H and cognitive functioning
Presence of T2H
Simple and pairwise comparisons. There were no significant cognitive differences between those with (T2H+) and without (T2H−) T2H for the presence of discrete lesions by either the simple or pairwise comparisons. For the simple comparison there were also no significant differences in cognition when taking into account the diffuse lesions (total T2H). Because of the small number of children without any T2H in the pairwise comparison group (n = 4), the power was not sufficient to conduct statistical analyses. T2H were not associated with the presence of either specific learning disabilities or ADHD. In general, the frequency of macrocephaly was not significantly different between the T2H− and T2H+ groups. Likewise, there were equal frequencies between the two groups for gender and sporadic/familial NF1. Those with T2 lesions (discrete and diffuse) were slightly younger on average (mean 11.4 years) than those without lesions (mean 13.3 years) (p = 0.029).
Number of T2H
Simple and pairwise comparisons. The number of T2H was not associated with performance on any cognitive test. There was however a significant negative correlation between age and number of discrete lesions for the larger NF1 sample (r = −0.288, p = 0.012).
Location of T2H
Simple comparison. There was no significant lowering of cognitive ability on any of the measures for children with discrete lesions in the basal ganglia (n = 45), brainstem (n = 16), corpus callosum (n = 10), cerebellum (n = 11) or hemispheres (n = 13) compared with children without lesions in these regions. For discrete thalamic lesions, the sample size was too small to warrant statistical testing (n = 6) but patients with discrete lesions in the thalamus showed low levels of cognitive functioning compared with those without lesions and with lesions elsewhere. The mean full scale intelligence quotient (FSIQ) for these patients was 72.8 compared with those without thalamic lesions who had a mean FSIQ of 91.4 (and controls with a mean of 102.6). They also performed much lower for Verbal IQ (VIQ), Performance IQ (PIQ), Verbal Comprehension Index, Perceptual Organisation Index (POI), Freedom From Distractibility and Processing Speed Index (PSI), with mean scores ranging from 72.0 to 84.0 (borderline range). They also performed over 1 SD below those without thalamic lesions for spelling, mathematics, verbal memory, Judgment of Line Orientation, switching attention, motor coordination and motor speed. Five of the six children were male. When diffuse lesions were also taken into account, the presence of lesions in the thalamus (total T2H) was associated with a poorer performance on switching attention (Z = −1.31, p = 0.013) and fine motor coordination (Z = −0.87, p = 0.036) compared with those without thalamic lesions (Z = −0.64; Z = −0.16, respectively).
Pairwise comparison. Discrete lesions in the basal ganglia (n = 27) and brainstem (n = 10) were not associated with a lowering of any cognitive ability. Owing to the small sample size of those with lesions in the cerebellum (n = 6), thalamus (n = 4), corpus callosum (n = 7) and hemispheres (n = 9), statistical analyses were not conducted because of lack of power. Qualitatively, patients with lesions in the cerebellum, corpus callosum and hemispheres had very similar difference scores to those with lesions elsewhere. However, lesions in the thalamus were associated with a lowering of FSIQ, VIQ, PIQ, Verbal Comprehension Index and POI in children with NF1 compared with their siblings. Those with discrete thalamic lesions performed 28.3 points lower on the FSIQ than their siblings, compared with those without thalamic lesions, which were only 9.0 points lower than their siblings. Similar differences were seen for many of the other cognitive tests, especially sustained attention, where those with thalamic lesions performed over 2 SD lower than their siblings compared with those without thalamic lesions which were within 1 SD of their siblings.
When examining total T2H (discrete plus diffuse T2H), lesions in the basal ganglia (n = 32), brainstem (n = 21), cerebellum (n = 25) and hemispheres (n = 10) were not associated with a lowering of either general intellectual functioning or any specific cognitive ability. Lesions in the corpus callosum (n = 9) were too few to calculate statistically, but qualitatively were similar to those without lesions. Lesions in the thalamus (n = 28) were associated with a lowering of FSIQ (p = 0.036), PIQ (p = 0.011), POI (p = 0.002) and PSI (p = 0.017). Children with T2H in the thalamus performed 14.4 points lower on the FSIQ compared with their siblings, 13.0 points lower on PIQ, 14.3 points on POI and 9.7 points on PSI.
Discussion
The results of the study show that discrete T2H are present in 71% of NF1 children whereas when diffuse lesions are also taken into account, T2H are present in 90% of children. T2H varied in terms of their frequency and intensity in different anatomical locations. Basal ganglia and hemispheric T2H were more likely to be discrete whereas lesions in the thalamus, cerebellum and brainstem were more likely to be diffuse. A relationship was found between age of the child with NF1 and the presence and number of T2H; those with T2H, on average, were younger than those without T2H, and a greater number of T2H were found in the younger children. This supports our previous longitudinal study showing that T2H disappear with age.32
The current study did not confirm a relationship between the presence or number of T2H and cognitive ability. We were unable to replicate the previous results from our own group and others that supported an association between the presence of T2H and cognitive dysfunction.10,11,12 This is probably because of differences in sample size, as inclusion/exclusion criteria and demographics of the NF1 cohorts have not varied markedly between studies and the current study has effectively double the sample size of our previous study.11 In addition, by using finer sections through the brain (volume T2 sequence), we have demonstrated that T2H may be comprised of a number of poorly delineated lesions. Thus lesion count can be affected by the sensitivity of the imaging technique used, and previous analyses correlating the number of lesions with cognitive ability may not be particularly accurate. We have addressed this possibility by performing a separate analysis of discrete lesions (which would be detected using less sensitive MRI techniques) and diffuse lesions (which could be missed or not counted as T2H).
Our data add to a growing body of research supporting a relationship between thalamic lesions and cognitive dysfunction in children with NF1. While discrete lesions in the thalamus are relatively rare (∼8%), they appear to have an extreme impact on the NF1 child's level of general intellectual functioning, with a lowering of over 18 IQ points compared with those without discrete thalamic lesions. When familial and other genetic factors were taken into account by examining the lowering of cognitive ability in NF1 children compared with their unaffected siblings, the four pairs of NF1 siblings with discrete thalamic lesions had extreme differences in cognitive ability, with the NF1 children having a mean IQ 28 points lower than their siblings, and falling over 2 SD below their siblings on a task of sustained attention. The presence of both discrete and diffuse lesions in the thalamus (present in 28/45 pairs) revealed a significant, but less severe, lowering of FSIQ, PIQ and processing speed when comparing the NF1 children with their siblings. Examining the lowering of cognitive ability of children with NF1 compared with their siblings appears to be more sensitive in detecting the effects of the NF1 gene on cognition than by comparing group means.
One previous study of children with NF1 found a strong association between T2H in the thalamus and IQ, memory, motor skills and attention.15 There is also evidence of a relationship between T2H in the thalamus and FSIQ and VIQ,14 as well as between FSIQ and T1 in the nucleus pulvinarus.33 In addition, lesions in the thalamus are more likely to evolve into true masses compared with T2H in several other regions, suggesting that lesions in the thalamus may be pathologically different to the T2H lesions seen elsewhere.34 Children with NF1 have decreased metabolism in the thalamus independent of the presence of T2H, suggesting that delays in thalamic signal processing may contribute to the neuropsychological deficits.35 Magnetic resonance spectroscopy has shown that NF1 patients had significantly less N‐acetylaspartate in the thalamus than healthy controls.36
It is therefore possible that lesions in the thalamus in conjunction with thalamic hypometabolism may compound the level of thalamic dysfunction, resulting in dysfunction well beyond that produced by T2H in other regions. Support for this hypothesis is evident in the present study, as discrete lesions in the thalamus were associated generally with more broad ranging cognitive deficits (with IQ, academic achievement, memory, perception and motor skills) as well as with more severe deficits (lower mean scores and greater difference scores between NF1 children and their siblings). When diffuse lesions were also taken into account, lesions in the thalamus were associated in general with attention and motor problems, and a lowering of IQ was seen in NF1 children compared with their siblings. However, the cognitive dysfunction in general was less widespread and severe than seen with the discrete lesions alone. This hypothesis has also been suggested by one other research study that found that smaller thalamic lesions were associated with cognitive problems.14 The authors postulated that as regressing lesions are associated with high choline and low N‐acetylaspartate levels, the regressing (ie, smaller) lesions will be associated with high cell turnover and neuronal loss, leading to deterioration in cognitive functioning. It is also possible that a different underlying pathology of T2H in the thalamus may be causative of the cognitive dysfunction. While there does not appear to be a relationship between changes in T2H and changes in cognitive ability,32 further longitudinal research examining whether changes in the thalamus are associated with changes in cognitive ability is warranted.
Abbreviations
ADHD - attention deficit–hyperactivity disorder
FLAIR - fluid attenuated inversion recovery
FSIQ - full scale intelligence quotient
NF1 - neurofibromatosis type 1
PIQ - Performance IQ
POI - Perceptual Organisation Index
PSI - Processing Speed Index
T2H - T2 hyperintensities
T2V - volume T2
VIQ - Verbal IQ
Footnotes
Funding: This research was supported by the Department of Defense Neurofibromatosis Research Program (managed by the US Army Medical Research and Materiel Command) as well as the National Neurofibromatosis Foundation, USA.
Competing interests: None.
References
- 1.Huson S M, Hughes R A C.The neurofibromatoses: A pathogenetic and clinical overview. London: Chapman and Hall, 1994
- 2.Hyman S L, Shores E A, North K N. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology 2005651037–1044. [DOI] [PubMed] [Google Scholar]
- 3.North K. Neurofibromatosis type 1. Am J Med Genet 200097119–127. [DOI] [PubMed] [Google Scholar]
- 4.Sevick R J, Barkovich A J, Edwards M S.et al Evolution of white matter lesions in neurofibromatosis type 1: MR findings. AJR Am J Roentgenol 1992159171–175. [DOI] [PubMed] [Google Scholar]
- 5.Dunn D W, Roos K L. MRI evaluation of learning difficulties and incoordination in neurofibromatosis type 1. Neurofibromatosis 198921–5. [PubMed] [Google Scholar]
- 6.Duffner P K, Cohen M E, Seidel F G.et al The significance of MRI abnormalities in children with neurofibromatosis. Neurology 198939373–378. [DOI] [PubMed] [Google Scholar]
- 7.Ferner R E, Chaudhuri R, Bingham J.et al MRI in neurofibromatosis 1. The nature and evolution of increased intensity T2 weighted lesions and their relationship to intellectual impairment. J Neurol Neurosurg Psychiatry 199356492–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Legius E, Descheemaeker M J, Spaepen A.et al Neurofibromatosis type 1 in childhood: a study of the neuropsychological profile in 45 children. Genet Couns 1994551–60. [PubMed] [Google Scholar]
- 9.Bawden H, Dooley J, Buckley D.et al MRI and nonverbal cognitive deficits in children with neurofibromatosis 1. J Clin Exp Neuropsychol 199618784–792. [DOI] [PubMed] [Google Scholar]
- 10.Feldmann R, Denecke J, Grenzebach M.et al Neurofibromatosis type 1. Motor and cognitive function and T2‐weighted MRI hyperintensities. Neurology 2003611725–1728. [DOI] [PubMed] [Google Scholar]
- 11.North K, Joy P, Yuille D.et al Learning difficulties in neurofibromatosis 1: The significance of MRI abnormalities. Neurology 199444878–883. [DOI] [PubMed] [Google Scholar]
- 12.Samango‐Sprouse C. Frontal lobe development in childhood. In: Miller BL, Cummings JL, eds. The human frontal lobes: Functions and disorders. The science and practice of neuropsychology series. New York: The Guilford Press, 1999589–592.
- 13.Denckla M B, Hofman K, Mazzocco M M.et al Relationship between T2‐weighted hyperintensities (unidentified bright objects) and lower IQs in children with neurofibromatosis‐1. Am J Med Genet 19966798–102. [DOI] [PubMed] [Google Scholar]
- 14.Goh W H, Khong P L, Leung C S.et al T2‐weighted hyperintensities (unidentified bright objects) in children with neurofibromatosis 1: Their impact on cognitive function. J Child Neurol 200419853–858. [DOI] [PubMed] [Google Scholar]
- 15.Moore B D, Slopis J M, Schomer D.et al Neuropsychological significance of areas of high signal intensity on brain MRIs of children with neurofibromatosis. Neurology 1996461660–1668. [DOI] [PubMed] [Google Scholar]
- 16.Gill D, Hyman S L, Steinberg A.et al Age‐related findings on MRI in neurofibromatosis type 1. Pediatr Radiol 2006361048–1056. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D.Wechsler Intelligence Scale for Children, 3rd edn. New York: The Psychological Corporation 1991
- 18.Wechsler D.Wechsler Individual Achievement Test. New York: The Psychological Corporation, 1992
- 19.Manly T, Robertson I H, Anderson V.et alThe Test of Everyday Attention for Children. London: Battley Brothers, 1999
- 20.Conners C K.Conners' Rating Scales‐Revised. New York: Multi‐ Health Systems Inc, 1997
- 21.Boll T.Children's Category Test. San Antonio: The Psychological Corporation, 1997
- 22.Krikorian R, Bartok J, Gay N. Tower of London procedure: A standard method and developmental data. J Clin Exp Neuropsychol 199416840–850. [DOI] [PubMed] [Google Scholar]
- 23.Yeudall L T, Fromm D, Reddon J R.et al Normative data stratified by age and sex for 12 neuropsychological tests. J Clin Psychol 198643918–946. [DOI] [PubMed] [Google Scholar]
- 24.Benton A, Varney N, Hamsher K.Judgement of Line Orientation. Iowa City: Department of Neurology, University of Iowa, 1976
- 25.Meyers J E, Meyers K R.Rey Complex Figure Test and Recognition Trial. Odessa, FL: Psychological Assessment Resources, 1995
- 26.Trahan D E, Larrabee G J.Continuous Visual Memory Test (CVMT). USA: Psychological Assessment Resources, 1988
- 27.Delis D C, Kramer J, Kaplan E.et alCalifornia Verbal Learning Test for Children (CVLT‐C). San Antonio: The Psychological Corporation, 1994
- 28.Trites R.Grooved Pegboard Instruction Manual. Lafayette, IN: Lafayette Instrument, 1989
- 29.Greenberg L M.Test of Variables of Attention 5.01. St Paul. MN: Attention Technology Inc, 1993
- 30.Hofman K J, Harris E L, Bryan R N.et al Neurofibromatosis type 1: the cognitive phenotype. J Pediatr 1994124S1–S8. [DOI] [PubMed] [Google Scholar]
- 31.Hyman S L, Shores E A, North K N. Learning disabilities in children with neurofibromatosis type 1: Subtypes, cognitive profile and ADHD. Dev Med Child Neurol 200648973–977. [DOI] [PubMed] [Google Scholar]
- 32.Hyman S L, Gill D, Shores E A.et al Natural history of neuropsychological ability and T2‐hyperintensities in patients with neurofibromatosis type 1. Neurology 2003601139–1145. [DOI] [PubMed] [Google Scholar]
- 33.Steen R G, Taylor J S, Langston J W.et al Prospective evaluation of the brain in asymptomatic children with neurofibromatosis type 1: relationship of macrocephaly to T1 relaxation changes and structural brain abnormalities. Am J Neuroradiol 200122810–817. [PMC free article] [PubMed] [Google Scholar]
- 34.DiMario F J, Ramsby G. Magnetic resonance imaging lesion analysis in neurofibromatosis type 1. Arch Neurol 199855500–505. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan A M, Chen K, Lawson M A.et al Positron emission tomography in children with neurofibromatosis‐1. J Child Neurol 199712499–506. [DOI] [PubMed] [Google Scholar]
- 36.Wang P Y, Kaufmann W E, Koth C W.et al Thalamic involvement in neurofibromatosis type 1: Evaluation with proton magnetic resonance spectroscopic imaging. Ann Neurol 200047477–484. [PubMed] [Google Scholar]