Abstract
Objective
To compare the clinical aspects of peripheral neuropathy associated with Wegener's granulomatosis (WG), Churg–Strauss syndrome (CSS) and microscopic polyangiitis (MP).
Methods
Cohort study conducted in a single university hospital. Patients were included when a definite diagnosis of WG, CSS or MP was made according to the current classification criteria in our hospital, between 1999 and 2006. All patients underwent periodically clinical and electrophysiological screening for peripheral neuropathy, assessment of disability, and clinical and laboratory evaluation during a mean follow‐up of 38 months.
Results
Sixty‐four consecutive patients diagnosed with WG (26 patients), CSS (26 patients) and MP (12 patients) were recruited. Peripheral neuropathy occurred in 27/64 patients: six with WG, 15 with CSS and six with MP. Neuropathy occurred earlier in the disease history in CSS and MP compared with WG. Among patients with WG, those who developed peripheral neuropathy during follow‐up were older than those without neuropathy both at the time of onset and of diagnosis of vasculitis. Distal symmetric polyneuropathy was present in 11 patients, and single or multiple mononeuropathy in 16. Patients with WG had a less severe form of mononeuritis multiplex than CSS or MPA patients. Disability and pain were greater in patients with mononeuropathy, although one‐third of them were painless. Relapses of neuropathy were extremely infrequent.
Conclusions
Peripheral neuropathy in WG occurs less frequently, later in the disease course and in a milder form than in CSS and MP. Single or multiple mononeuropathy associated with these subsets of vasculitis can often be painless.
Wegener's granulomatosis (WG), Churg–Strauss syndrome (CSS) and microscopic polyangiitis (MP) constitute a group of small vessel systemic vasculitides (SVSVs), involving preferentially venules, capillaries and arterioles, that share common histological features. They are often referred to as “pauci‐immune” SVSVs, because of the scarceness of immune complex deposition, or as “ANCA associated” SVSVs because antineutrophil cytoplasmic antibodies (ANCA) are often found in the serum of patients.1,2 Despite this nomenclature, ANCA may be absent in some patients in all three subsets, but more often in CSS. While the diagnostic relevance of ANCA is widely accepted, there is still debate on their possible, if any, pathogenetic role.3,4,5,6 The main distinctive features of the three subsets include: necrotising granulomatous inflammation in the absence of asthma for WG, asthma, eosinophilia and necrotising granulomatous inflammation for CSS and absence of both granulomatous inflammation and asthma for MP.
SVSVs can affect virtually any organ system in the body, resulting in a wide variety of signs and symptoms. The peripheral nervous system represents no exception, being frequently involved, and may also be an initial manifestation of the clinical picture.7,8 Pathogenesis is linked to a primary process of inflammation and of the vessel wall, resulting in blood flow impairment and, ultimately, in ischaemia of the supplied tissues. However, vasculitic processes with very similar histological features and anatomical distribution may determine heterogeneous clinical pictures and outcomes.9,10 In this study, we evaluated 64 consecutive patients diagnosed with pauci‐immune SVSV in order to determine differences in the involvement of the peripheral nervous system in the three subsets.
Methods
Inclusion criteria
The study was conducted on a cohort of consecutive patients in internal medicine departments (nephrology, clinical immunology, rheumatology, pulmonology, neurology and others) in one general hospital in Parma, Italy, within our interdisciplinary Secondary and Primary Vasculitides Study Group,11 between January 1999 and November 2006. All patients gave informed consent to the procedures described in the present work. Patients were included in the study when a definite diagnosis of vasculitis was made and the criteria for classification in one of the three subsets were met. WG and CSS were classified according to the American College of Rheumatology criteria12,13 and MP on the basis of the Chapel Hill Consensus Conference definition14 and according to the clinical and serological picture. Patients were excluded from the cohort if other known causes of neuropathy were present, such as diabetes mellitus, alcoholism, hypothyroidism, paraproteinaemia, human immunodeficiency virus infection, hepatitis C virus associated cryoglobulinaemia, Lyme disease, heavy metal intoxication, cobalamine or folic acid deficiency, coeliac disease, cancer and familial neuropathies. Patients were excluded during follow‐up if an alternative or associated diagnosis was suggested by new clinical and laboratory findings.
Clinical evaluation
Each patient was evaluated at the time of the diagnosis of systemic vasculitis and periodically during a mean follow‐up of 37 months (6–88 months), regardless of the presence of clinical signs of peripheral neuropathy. They underwent at each visit: (1) clinical evaluation by an experienced neurologist; (2) electrophysiological studies; (3) semiquantitative evaluation of the functional impairment due to peripheral neuropathy by a functional Disability Score using a five point scale8,15 (0 = no neurological symptoms or signs; 1 = symptoms but no signs or vice versa; 2 = mild motor or sensory symptoms or signs; 3 = moderately disabled by motor and sensory symptoms including ataxia; 4 = requires assistance with activities of daily living or uses a walking aid; 5 = not walking); and (4) assessment of disease activity using the Birmingham Vasculitis Activity Score (BVAS) type 1.16 We subtracted the peripheral nervous system items from the BVAS for statistical analysis concerning peripheral neuropathy. ANCA were determined at the time of diagnosis, before starting immunosuppressive treatment, using indirect immunofluorescence on ethanol fixed granulocytes and antigen specific PR3 and MPO ELISAs.17
The diagnosis of SVSV was made in all patients in our institution. In most patients the onset of disease had been recorded in our institution but in nine patients the time of disease onset was reconstructed retrospectively.
Electrophysiological examination and diagnosis of peripheral neuropathy
Nerve conduction studies were performed using standard techniques of surface stimulation and recording. Screening electrophysiological examination, that was performed in all subjects in the cohort at each visit, consisted of the following: motor nerve conduction studies were performed in both lower extremities on the peroneal nerve and in one upper extremity on the median nerve. Sensory nerve conduction studies were performed on both sural nerves and on median and ulnar nerves on one side. Electroneurographic data were compared with normative values of our laboratory. In addition, needle electromyography was performed on distal muscles of the lower limbs, notably the extensor digitorum brevis and the tibialis anterior muscles. In symptomatic cases, both nerve conduction studies and needle EMG were extended to the appropriate nerves and muscles, as suggested by the clinical picture or by electrophysiological abnormalities. The final diagnosis of peripheral neuropathy was made on the basis of both electrophysiological signs and clinical signs and symptoms.
Neuropathy was classified as distal symmetric polyneuropathy (PNP) or as single or multiple mononeuropathy (MN). PNP was diagnosed when a patient showed both clinical and electrophysiological abnormalities with a bilateral diffuse length dependent topography, therefore principally in the distal lower limbs with possible extension to the upper limbs. Some degree of asymmetry was tolerated. More in detail, to be diagnosed with PNP, a patient was required to show the following signs: reduced or absent ankle reflexes and distal reduced or absent vibration sense (128 Hz tuning fork) and/or superficial tactile sensation (cotton wisp) with or without decreased pinprick sensation in the lower limbs. Distal muscle weakness or atrophy was not considered necessary to diagnose PNP. Additionally, at least one positive (tingling, pain or allodynia) or negative (numbness, hypoesthesia) symptom had to be present in the extremities. Finally, a collection of electroneurographic abnormalities had to be present: bilateral amplitude reduction or absence of sensory and muscle action potentials or of sensory action potentials alone with or without alterations of conduction velocity. When only sensory action potentials were reduced, the finding in sural nerves was required to be confirmed in the superficial peroneal nerves. An isolated increase in distal motor latency alone was not considered as an abnormality. Needle EMG was confirmatory but not diagnostic of PNP and was considered useful in establishing the mainly axonal type of nerve damage. Our diagnostic criteria for PNP therefore fulfilled the recently proposed highest likelihood criteria for the diagnosis of distal symmetric polyneuropathy.18
Single or multiple mononeuritis was diagnosed in the presence of medium to complete weakness in the muscles supplied by single nerves, associated with medium to severe sensation loss in the territory of the same nerve. Sensory symptoms were not required for the diagnosis. The electrophysiological pattern was required to show impairment of individual nerves. In this case, needle EMG was of high diagnostic importance in showing neuropathic changes and pathological spontaneous activity in the muscles supplied by the affected nerve and demonstrating normal findings in the muscles innervated by other anatomically contiguous nerves. The clinical picture and patient history were helpful in distinguishing extensive confluent MN in the lower limbs from PNP and in excluding coexistent entrapment mononeuropathies unrelated to the vasculitic process. In this respect, neuropathy of the median nerve at the wrist was never considered as vasculitic in origin, because of the high prevalence of carpal tunnel syndrome in the general population. Also, cranial neuropathies were not considered as peripheral neuropathy (see discussion). The onset of neuropathy was classified as acute or chronic if the initial progression of the symptoms was less than or greater than 1 month.8
Statistical analysis
The significance level was set at 0.05. Statistical significance between disease subsets and between patients with or without neuropathy was assessed using univariate factorial ANOVA. The variables that were tested were: (a) age at onset, (b) age at diagnosis, (c) time interval between vasculitis onset or diagnosis and onset of neuropathy and (d) the BVAS. All variables were tested for ANOVA assumptions. Post hoc analyses were made with Bonferroni corrected t tests. The analysis of nominal variables was made with a χ2 test. Within‐subject variables were compared using the t test for paired data. In the text and tables, mean (SEM) values are represented.
Results
A clinical summary of the population is presented in table 1. Sixty‐five patients fulfilled the inclusion criteria. Twenty‐six patients were diagnosed with CSS12 and 26 with WG.13 In the remaining 13 patients, the diagnosis of MP was made. One patient with MP was excluded during follow‐up because an additional diagnosis of rheumatoid arthritis was made on the basis of new clinical and laboratory findings. The final cohort therefore comprised 64 patients. Histological confirmation of the diagnosis was obtained in 28 cases (12 with WG, 14 with CSS and three with MP) from the appropriate organs according to the clinical picture, including upper and lower respiratory tract, skin, kidney and gastrointestinal mucosa. Peripheral nerve and muscle were sampled in one subject.
Table 1 Demographical, clinical and laboratory data.
| Patients without neuropathy | Patients with neuropathy | |||||
|---|---|---|---|---|---|---|
| WG | CSS | MP | WG | CSS | MP | |
| No of patients (M/F) | 20 (9/11) | 11 (1/10) | 6 (2/4) | 6 (3/3) | 15 (11/4) | 6 (1/5) |
| Age at vasculitis onset (y) | 41.5 (2.6) | 47.1 (4.1) | 60.1 (6.3) | 59.6 (1.0) | 50.1 (3.1) | 60.0 (3.2) |
| Age at vasculitis diagnosis (y) | 43.1 (2.6) | 50.0 (3.8) | 60.9 (5.0) | 64.6 (2.9) | 49.3 (2.9) | 65.2 (1.9) |
| BVAS‐n | 11.8 (1.7) | 6.3 (1.1) | 16.5 (1.3) | 14.0 (3.4) | 6.1 (1.1) | 11.0 (2.7) |
| cANCA positive patients | 14 | 0 | 1 | 6 | 2 | 0 |
| pANCA positive patients | 1 | 2 | 5 | 0 | 6 | 6 |
| Follow‐up (months) | 36.8 (4.4) | 32.1 (4.9) | 36.7 (4.7) | 42.7 (8.1) | 37.6 (3.7) | 41.3 (7.4) |
ANCA, antineutrophil cytoplasmic antibody; BVAS‐n, Birmingham Vasculitis Activity Score, subtracted from the peripheral nervous system items; cANCA, cytoplasmic ANCA; CSS, Churg–Strauss syndrome; MP, microscopic polyangiitis; pANCA, perinuclear ANCA; WG, Wegener's granulomatosis.
Peripheral neuropathy occurred in 27 of 64 patients. Four additional patients were found to have bilateral reduction in sural nerve sensory action potentials but were completely asymptomatic and neurological examination was not significant. These cases were considered not to have peripheral neuropathy, and follow‐up assessments did not show any change in the electrophysiological parameters. The distribution of patients with and without neuropathy in the three disease groups was significant (χ2(2) = 6.76, p<0.05) because of the fact that neuropathy was less frequent in WG than in CSS when comparing only these two groups (χ2(1) = 6.47, p<0.05). ANCA were not significantly associated with neuropathy in the general population or in any of the three subsets. Neuropathy was more frequent in male than in female patients (χ2(1) = 10.53, p<0.01) only in the CSS group.
Age at onset and at diagnosis of systemic vasculitis
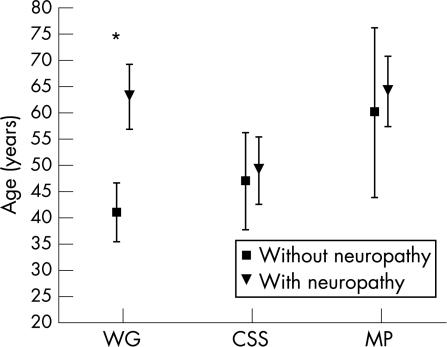
Age at onset of vasculitis was significantly different in the three groups (F(2, 58) = 5.6794, p<0.01), being greater in patients with MP (61.8 (3.3) years) than in those with WG (46.5 (2.8) years, p = 0.001) and CSS (48.1 (2.4) years, p<0.005). A significant interaction between disease subset and the presence of neuropathy was found (F(2,58) = 4.2862, p<0.05) because of the fact that, only in the WG group, patients who developed peripheral neuropathy were older at the onset of systemic disease than those who did not develop neuropathy (p<0.005) (table 1, fig 1).
Figure 1 Mean age at onset of systemic disease of patients in the three disease groups who did or did not develop peripheral neuropathy during follow‐up. Values are mean (95% CI). CSS, Churg–Strauss syndrome; MP, microscopic polyangiitis; WG, Wegener's granulomatosis. *p<0.005.
Also, age at diagnosis of vasculitis was differently distributed between patients in the three subsets (F(2,58) = 5.9449, p = 0.005) and was significantly higher in patients with MP (mean age 63.1 (2.7) years) than in those with WG (mean age 48.1 (2.8) years, p = 0.0001) or CSS (mean age 49.6 (2.3) years, p<0.005). A significant interaction between disease subset and presence of neuropathy was found (F(2,58) = 5.4705, p<0.01), because of the fact that WG patients who developed neuropathy were diagnosed at an older age than patients without neuropathy (p<0.005).
Time course of peripheral neuropathy
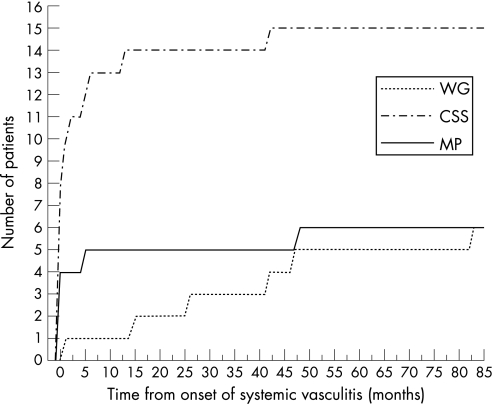
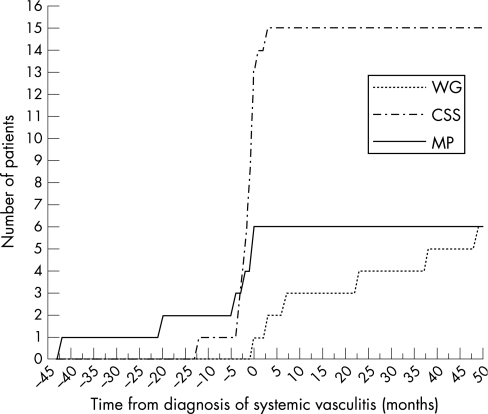
In 16 of 27 patients, neuropathy represented one of the first clinical manifestations of systemic vasculitis, occurring within 2 months from the onset of systemic disease (one with WG, 11 with CSS, and four with MP) (see fig 2). Among these 16 patients, in eight cases neuropathy preceded any other organ involvement by at least 2 months. The mean interval between the onset of systemic vasculitis and the onset of peripheral neuropathy was significantly different in the three subsets (F(2, 24) = 6.6118, p = 0.005), being higher in patients with WG (36.2 (12.1) months) compared with patients with CSS (4.6 (2.8) months, p<0.005) and MP (9.0 (7.9) months, p<0.05). Also, the interval between the diagnosis of vasculitis and the onset of peripheral neuropathy was different between the three subsets (F(2, 24) = 10.385, p<0.001). Patients with WG developed peripheral neuropathy significantly later in relation to the time of diagnosis of vasculitis (mean interval between diagnosis and onset of neuropathy 19.9 (8.2) months compared with CSS (−1.4 (0.8) months, p<0.005) and MP (mean −11.2 (6.9) months, p<0.001); the difference between the latter two were not significant (fig 3).
Figure 2 Time course of the manifestation of peripheral neuropathy from the onset of systemic disease (time = 0) in the three groups. CSS, Churg–Strauss syndrome; MP, microscopic polyangiitis; WG, Wegener's granulomatosis.
Figure 3 Time course of the manifestation of peripheral neuropathy in relation to the moment of diagnosis of systemic disease. CSS, Churg–Strauss syndrome; MP, microscopic polyangiitis; WG, Wegener's granulomatosis.
Clinical and electrophysiological features of peripheral neuropathy
Clinical data of patients with peripheral neuropathy are shown in table 2. No significant association was found between the type of neuropathy (PNP or MN) and the disease subset, age of onset, time of onset with respect to diagnosis, onset modality (acute or chronic) and ANCA presence and pattern. Pain was significantly associated with MN compared with PNP (χ2(1) = 5.19, p<0.05), although MN was painless in 6/16 patients. Moreover, when considering the pool of individual nerves affected in MN, 17 of 53 (32%) were painless. Patients were considered to have painless neuropathy if pain was absent throughout the follow‐up period. Among patients with MN, the number of affected nerves throughout the follow‐up period was significantly less in WG than the number of nerves affected in patients with CSS or MP (p<0.05), as shown in table 1. The most frequently involved nerve was the peroneal (14 patients, 23 nerves), followed by the tibial (10 patients, 16 nerves), median (five patients, five nerves), ulnar (four patients, four nerves), radial (three patients, three nerves) and the sciatic (two patients, two nerves) nerves. Electrophysiological studies showed a pattern of abnormalities consistent with axonal damage in all patients. Four patients presented with cranial neuropathy. One patient had WG, showing unilateral third nerve palsy in the context of a granulomatous pachimeningitis. Two patients had CSS, one showed a unilateral hypoglossal palsy and in the other the fourth cranial nerve was involved, associated with a contiguous orbital granuloma. One patient had MP and showed unilateral seventh cranial nerve palsy. Except for the patient with pachimeningitis, the remaining three patients also had peripheral neuropathy apart from cranial nerve involvement.
Table 2 Clinical features of patients with peripheral neuropathy.
| Mononeuropathy or mononeuropathy multiplex | Distal symmetric polyneuropathy | |||||
|---|---|---|---|---|---|---|
| WG | CSS | MP | WG | CSS | MP | |
| No of patients (M/F) | 3 (3/0) | 9 (7/2) | 4 (0/4) | 3 (0/3) | 6 (4/2) | 2 (1/1) |
| cANCA positive patients | 3 | 0 | 0 | 3 | 2 | 0 |
| pANCA positive patients | 0 | 3 | 4 | 0 | 3 | 2 |
| Painful neuropathy (n) | 0 | 7 | 3 | 1 | 0 | 1 |
| No of involved nerves | 1.0 (0.0) | 3.9 (0.6) | 3.8 (0.5) | – | – | – |
ANCA, antineutrophil cytoplasmic antibody; cANCA, cytoplasmic ANCA; CSS, Churg–Strauss syndrome; MP, microscopic polyangiitis; pANCA, perinuclear ANCA; WG, Wegener's granulomatosis.
Follow‐up and prognosis
All patients were treated with corticosteroids and cyclophosphamide during periods of disease activity. Clinical and electrophysiological evaluations during follow‐up revealed both an improvement in motor deficits and a reduction in sensory deficits. Only one patient with WG had a relapse of the neuropathy after a period of 4 years. Three patients died and one had peripheral neuropathy. The mean Disability Score of patients with peripheral neuropathy was significantly improved (p = 0.001) between the first (2.4 (0.26)) observation and the last observation (1.3 (0.21)). The mean Disability Score at the first observation was significantly higher in patients with MN (3.2 (0.31)) than in those with PNP (1.6 (0.20)) (p<0.0005) and this difference was not present at the last observation (1.4 (0.20) vs 1.1 (0.23)). BVAS at both the first and last observation was not significantly different between the three disease groups or between patients with or without neuropathy. In the whole population, BVAS was significantly decreased at the last observation compared with the first observation (p = 0.0001) (table 1).
Discussion
In this study, we provided a direct comparison of peripheral nervous system involvement in the three subsets of the so‐called “ANCA associated” SVSVs, as determined prospectively by electrophysiological examination. No selection was made prior to the neurophysiological examination, and all patients underwent immunosuppressive treatment. Therefore, the data on peripheral neuropathy are representative of populations of treated patients in the three vasculitis subsets. Our results confirm that neuropathy is a frequent clinical manifestation of SVSV, being documented in 42% of our series of 64 patients. These results are comparable with data reported in the literature, where the frequency of peripheral nervous system involvement varies from 11% to 44% in WG,8,19,20,21 from 43% to 72% in CSS22,23,24,25,26,27,28 and from 55% to 79% in patients with MP.29,30,31
In our series, however, we demonstrated that patients with WG developed peripheral neuropathy less frequently than patients with CSS and MP. Also, the clinical features and time course of peripheral neuropathy were considerably different in the three subsets of SVSVs. Among the patients with WG, those who developed a neuropathy during the follow‐up period were significantly older at the onset of disease compared with patients who did not develop neuropathy, as reported previously.8 This was true regardless of the duration of follow‐up and such a distinction was not found in patients with CSS or MP (fig 1). It was also observed that patients with WG developed the neuropathy later in the course of the disease compared with CSS and MP, and peripheral neuropathy was more often observed as an early manifestation of systemic vasculitis in CSS and MP (fig 2). Theses findings call for additional attention by neurologists, who are more likely to encounter neuropathies related to ANCA associated systemic vasculitides (AASVs) in patients with undiagnosed CSS or MP and less likely with WG. In the literature, an earlier onset of neuropathy has been described in CSS, but not in MP.32
MN occurred in a milder form in WG patients, being limited to one nerve per patient, compared with those with CSS or MP. Male patients with CSS in our population were more likely to develop a neuropathy, compared with female patients. No such difference was found in the WG or MP groups. A possible bias in this observation is the later onset of neuropathy in WG patients, meaning that it occurred when the patients were already under immunosuppressive treatment. The prevalence of the two forms of neuropathy, MN and PNP, was not different between the three subsets. This finding was only in partial agreement with the data in the literature, the reported ratio of MN to PNP in the three disease groups being highly variable. This is particularly relevant in CSS, where MN was the second most common clinical manifestation after asthma at disease onset in the two series,23,32 and it has been considered as the hallmark of vasculitic peripheral nerve involvement,24 being included in the classification criteria of the American College of Rheumatology.12 Cranial neuropathy was a rare event in our series, occurring in 6% of patients, and was considered separate from peripheral neuropathy because its pathogenesis can be more complex, involving contiguous infiltration or compression from adjacent granulomas.8 It is still debated whether a primitively demyelinating neuropathy can occur in the context of systemic vasculitis.8,33 In our population, electrophysiological studies showed a pattern compatible with axonal neuropathy in all patients.
The pathogenetic role, if any, of ANCA in the so‐called AASVs is still not clear. On the one hand, animal models and human case reports provide in vivo evidence that ANCA can be a transferable cause of disease.6 On the other hand, not all patients have these autoantibodies; some patients with high levels of autoantibodies have no evidence of active disease and ANCA are also found in other diseases without vasculitic complications, including hepatitis, or chronic inflammatory bowel diseases.34 Population studies show that the presence of ANCA and titre are statistically associated with peripheral neuropathy in WG8 and with MN in CSS.17,35 Conversely, in single cases it is already known that ANCA are not a reliable indicator of a vasculitic pathogenesis in patients with neuropathy.36 Unlike previous studies,17 we did not find any association of ANCA with peripheral neuropathy in our patients. This finding can be explained by either the smaller cohort in our study or by the methodological difference in the definition of peripheral nervous impairment. In fact, only one other study in the literature used systematic neurophysiological assessment in a cohort of patients with SVSV.8
The outcome of peripheral neuropathy secondary to AASVs was good, as approximately all patients achieved consistent recovery from the neurological disability and relapse of neuropathy was observed in only one patient. As reported previously,8 MN caused greater functional disability than PNP, although such differences disappeared at the last observation after treatment. Vasculitic neuropathy is commonly associated with pain.3 In our series, we found a high proportion of patients with painless neuropathy, mainly with PNP (table 2). However, among patients with MN about 1 in 3 were completely painless.
Acknowledgements
We acknowledge all the members of the Secondary and Primary Vasculitides Study Group (SePriVa) for preliminary clinical assessment of the patients.
Abbreviations
AASVs - antineutrophil cytoplasmic antibody associated systemic vasculitides
ANCA - antineutrophil cytoplasmic antibodies
BVAS - Birmingham Vasculitis Activity Score
CSS - Churg–Strauss syndrome
MN - mononeuropathy
MP - microscopic polyangiitis
PNP - polyneuropathy
SVSVs - small vessel systemic vasculitides
WG - Wegener's granulomatosis
Footnotes
Research funding: Luigi Cattaneo, Elisabetta Chierici and Chiara Grasselli were supported by a grant from the Italian Ministry of University and Research (MIUR)
Competing interests: None.
References
- 1.Jennette J C, Falk R J. Small‐vessel vasculitis. N Engl J Med 19973371512–1523. [DOI] [PubMed] [Google Scholar]
- 2.Savage C O, Harper L, Adu D. Primary systemic vasculitis. Lancet 1997349553–558. [DOI] [PubMed] [Google Scholar]
- 3.Schaublin G A, Michet C J, Jr, Dyck P J.et al An update on the classification and treatment of vasculitic neuropathy. Lancet Neurol 20054853–865. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman G S, Specks U. Antineutrophil cytoplasmic antibodies. Arthritis Rheum 1998411521–1537. [DOI] [PubMed] [Google Scholar]
- 5.Day C J, Hewins P, Savage C O. New developments in the pathogenesis of ANCA‐associated vasculitis. Clin Exp Rheumatol 200321S35–S48. [PubMed] [Google Scholar]
- 6.Pankhurst T, Savage C O. Pathogenic role of anti‐neutrophil cytoplasmic antibodies in vasculitis. Curr Opin Pharmacol 20066190–196. [DOI] [PubMed] [Google Scholar]
- 7.Hattori N, Ichimura M, Nagamatsu M.et al Clinicopathological features of Churg–Strauss syndrome‐associated neuropathy. Brain 1999122427–439. [DOI] [PubMed] [Google Scholar]
- 8.de Groot K, Schmidt D K, Arlt A C.et al Standardized neurologic evaluations of 128 patients with Wegener granulomatosis. Arch Neurol 2001581215–1221. [DOI] [PubMed] [Google Scholar]
- 9.Weyand C M, Goronzy J J. Multisystem interactions in the pathogenesis of vasculitis. Curr Opin Rheumatol 199793–11. [DOI] [PubMed] [Google Scholar]
- 10.Fietta P. Systemic vasculitides: immunogenetics and familial clustering. Clin Exp Rheumatol 200422238–251. [PubMed] [Google Scholar]
- 11.Pavone L, Grasselli C, Chierici E.et al Outcome and prognostic factors during the course of primary small‐vessel vasculitides. J Rheumatol 2006331299–1306. [PubMed] [Google Scholar]
- 12.Masi A T, Hunder G G, Lie J T.et al The American College of Rheumatology 1990 criteria for the classification of Churg–Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990331094–1100. [DOI] [PubMed] [Google Scholar]
- 13.Leavitt R Y, Fauci A S, Bloch D A.et al The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum 1990331101–1107. [DOI] [PubMed] [Google Scholar]
- 14.Jennette J C, Falk R J, Andrassy K.et al Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 199437187–192. [DOI] [PubMed] [Google Scholar]
- 15.Prineas J. Polyneuropathies of undetermined cause. Acta Neurol Scand Suppl 1970441–72. [DOI] [PubMed] [Google Scholar]
- 16.Luqmani R A, Bacon P A, Moots R J.et al Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. Q J Med 199487671–678. [PubMed] [Google Scholar]
- 17.Sinico R A, Di Toma L, Maggiore U.et al Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg–Strauss syndrome. Arthritis Rheum 2005522926–2935. [DOI] [PubMed] [Google Scholar]
- 18.England J D, Gronseth G S, Franklin G.et al Distal symmetrical polyneuropathy: definition for clinical research. Muscle Nerve 200531113–123. [DOI] [PubMed] [Google Scholar]
- 19.Fauci A S, Haynes B F, Katz P.et al Wegener's granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med 19839876–85. [DOI] [PubMed] [Google Scholar]
- 20.Nishino H, Rubino F A, DeRemee R A.et al Neurological involvement in Wegener's granulomatosis: an analysis of 324 consecutive patients at the Mayo Clinic. Ann Neurol 1993334–9. [DOI] [PubMed] [Google Scholar]
- 21.Drachman D D. Neurological complications of Wegener's granulomatosis. Arch Neurol 19638145–155. [Google Scholar]
- 22.Chumbley L C, Harrison E G, Jr, DeRemee R A. Allergic granulomatosis and angiitis (Churg–Strauss syndrome). Report and analysis of 30 cases. Mayo Clin Proc 197752477–484. [PubMed] [Google Scholar]
- 23.Guillevin L, Cohen P, Gayraud M.et al Churg–Strauss syndrome. Clinical study and long‐term follow‐up of 96 patients. Medicine (Baltimore) 19997826–37. [DOI] [PubMed] [Google Scholar]
- 24.Lanham J G, Elkon K B, Pusey C D.et al Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg–Strauss syndrome. Medicine (Baltimore) 19846365–81. [DOI] [PubMed] [Google Scholar]
- 25.Sehgal M, Swanson J W, DeRemee R A.et al Neurologic manifestations of Churg–Strauss syndrome. Mayo Clin Proc 199570337–341. [DOI] [PubMed] [Google Scholar]
- 26.Solans R, Bosch J A, Perez‐Bocanegra C.et al Churg–Strauss syndrome: outcome and long‐term follow‐up of 32 patients. Rheumatology (Oxford) 200140763–771. [DOI] [PubMed] [Google Scholar]
- 27.Della Rossa A, Baldini C, Tavoni A.et al Churg–Strauss syndrome: clinical and serological features of 19 patients from a single Italian centre. Rheumatology (Oxford) 2002411286–1294. [DOI] [PubMed] [Google Scholar]
- 28.Churg J C, Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol 195127277–294. [PMC free article] [PubMed] [Google Scholar]
- 29.Savage C O, Winearls C G, Evans D J.et al Microscopic polyarteritis: presentation, pathology and prognosis. Q J Med 198556467–483. [PubMed] [Google Scholar]
- 30.Agard C, Mouthon L, Mahr A.et al Microscopic polyangiitis and polyarteritis nodosa: how and when do they start? Arthritis Rheum 200349709–715. [DOI] [PubMed] [Google Scholar]
- 31.Guillevin L, Durand‐Gasselin B, Cevallos R.et al Microscopic polyangiitis: clinical and laboratory findings in eighty‐five patients. Arthritis Rheum 199942421–430. [DOI] [PubMed] [Google Scholar]
- 32.Lane S E, Watts R A, Shepstone L.et al Primary systemic vasculitis: clinical features and mortality. Q J Med 20059897–111. [DOI] [PubMed] [Google Scholar]
- 33.McCluskey L, Feinberg D, Cantor C.et al “Pseudo‐conduction block” in vasculitic neuropathy. Muscle Nerve 1999221361–1366. [DOI] [PubMed] [Google Scholar]
- 34.Hansch G M, Iking‐Konert C, Andrassy K. The pathogenesis of ANCA‐associated vasculitides: old hypotheses and new insights. Clin Nephrol 200564460–464. [DOI] [PubMed] [Google Scholar]
- 35.Sable‐Fourtassou R, Cohen P, Mahr A.et al Antineutrophil cytoplasmic antibodies and the Churg–Strauss syndrome. Ann Intern Med 2005143632–638. [DOI] [PubMed] [Google Scholar]
- 36.Chalk C H, Homburger H A, Dyck P J. Anti‐neutrophil cytoplasmic antibodies in vasculitis peripheral neuropathy. Neurology 1993431826–1827. [DOI] [PubMed] [Google Scholar]