Abstract
Aim
Neuropathological examination of both individuals in a monozygotic (MZ) twin pair with Alzheimer's disease (AD) is rare, especially in the molecular genetic era. We had the opportunity to assess the concordance and discordance of clinical presentation and neuropathology in three MZ twin pairs with AD.
Methods
The MZ twins were identified and characterised by the University of Washington Alzheimer's Disease Research Center. We reviewed the available clinical and neuropathological records for all six cases looking specifically for concordance and discordance of clinical phenotype, neuritic amyloid plaques (NP), neurofibrillary tangles (NFT) and Lewy related pathology (LRP).
Results
Discordance in age of onset for developing AD in the MZ twins varied from 4 to 18 years. Clinical presentations also differed between twins. One twin presented with a dementia with Lewy Body clinical syndrome while the other presented with typical clinical AD. Neuropathology within the MZ twin pairs was concordant for NP and NFT, regardless of duration of disease, and was discordant for LRP. This difference was most marked in the late onset AD twin pair. One pair was found to have a mutation in presenilin‐1 (PS1) (A79V) with remarkably late onset in a family member.
Conclusions
MZ twins with AD can vary considerably in age of onset, presentation and disease duration. The concordance of NP and NFT pathological change and the discordance of LRP support the concept that, in AD, the former are primarily under genetic control whereas the latter (LRP) is more influenced by disease duration and environmental factors. The A79V mutation in PS1 can be associated with very late onset of dementia.
Alzheimer's disease (AD) is the most common neurodegenerative disorder causing dementia in the elderly. AD has typically been divided into early onset (EOAD, onset <60 years) and late onset (LOAD, onset ⩾60 years). LOAD occurs in the majority (>95%) of patients with AD1 while EOAD accounts for a small proportion of patients (<5%). Demarcation of familial LOAD/EOAD is not absolute as approximately 25% of families with familial LOAD will have members with EOAD, accounting for about 6% of all affected cases.2
The cause of AD is unknown in the majority of cases. The most powerful risk factors for developing LOAD are age, a positive family history and APOE genotype.3
Specific mendelian genetic factors have also been identified in patients who develop familial EOAD, namely mutations in presenilin 1 (PS1),4 presenilin 2 (PS2)5 and amyloid precursor protein.6 PS1 mutations are the most frequent pathogenic mutation in EOAD.7
Genetic variation influences a number of aspects of clinical phenotype in patients affected by AD,1 with modelling suggesting that environmental factors are also important in determining development of dementia.8 Monozygotic (MZ) twins provide a unique insight into the relationship between genetic programming and environmental factors impacting on that programming. Twin studies suggest a polygenic multifactorial mode of inheritance1 and heritability for (LO)AD, being somewhere between 58% and 79%.9 Concordance rates in MZ twins can be as high as 61%,9 with concordance rates increasing with increasing duration of follow‐up.10 In a recent identical twin study, the environmental risk factors that predicted the development of (LO)AD were a history of tooth loss before 35 years (presumably reflecting childhood deprivations) and low educational attainment.11
Neuritic amyloid plaques (NP) and neurofibrillary tangles (NFT) are the neuropathological hallmarks of AD, with NFT composed of the microtubule associated protein tau. Alpha‐synuclein (SNCA) is a major component of Lewy related pathology (LRP, including inclusions and neuritic pathological change) and is a presynaptic protein which may be involved in synaptic function.12 LRP is a pathological hallmark of “idiopathic” Parkinson's disease but it can also be found in up to 60% of sporadic AD and approximately 5% of elderly, non‐parkinsonian individuals.13
There are few data in the literature on the concordance of neuropathology in MZ twins with AD. To our knowledge, only three case reports that included neuropathology of both twins in a pair with AD have been published14,15,16 and these were before the advent of beta amyloid, tau and SNCA immunohistochemistry and genetic testing. Age at onset of AD in two of these case reports (age of onset 3414 and 50 years16) raises the possibility that specific genetic mutations could have been responsible for the disease. Other reports of neuropathology in affected twins have included only one autopsy per twin pair17 or have not commented on neuropathology details.10 We are unaware of any case reports of autopsies in both twins of a MZ pair with a PS1 mutation.
We had the unique opportunity of identifying three kindreds with affected MZ twin pairs, in which neuropathology was available in all six cases and one pair had a mutation in PS1. This allowed us to review the clinical information, genetic status and detailed neuropathology on all six subjects to assess the concordance and discordance for all of these variables.
Methods
Three kindreds with male MZ twins were identified from pedigrees characterised by the University of Washington Alzheimer's Disease Research Center using protocols approved by the Human Subjects Research Office (IRB). Concordance and discordance of clinical history and neuropathological findings were assessed.
The diagnosis of AD was based on clinical examinations of affected individuals, whenever possible, as well as medical records and family history. Clinical criteria for AD were those suggested by McKhann and colleagues.18 Age of onset was determined to be that age at which family members and records agreed that the individual first began showing signs of memory loss or behavioural changes.19 As age of onset estimations are always somewhat arbitrary, age at death was also analysed as an endpoint.
Neuropathology
Neuropathological evaluations were performed at the University of Washington Medical Center, Seattle, by neuropathologists from the Alzheimer's Disease Research Center. Neuropathological examinations focused on the cingulate gyrus; superior and middle frontal gyri; medial orbital cortex; superior, middle and inferior temporal gyri; inferior parietal lobule; medial occipital cortex; hippocampus; amygdala; parahippocampal gyrus; hypothalamus; thalamus; midbrain; pons; medulla; and cerebellum. Standard tissue histological staining consisted of haematoxylin–eosin, thioflavin‐S and modified Bielschowsky silver staining on 8 μm thick paraffin embedded sections. Braak staging for NFT pathology20 and the Consortium to Establish a Registry for Alzheimer's Disease staging for senile plaque pathology21 were based on evaluation of modified Bielschowsky stained sections.
Immunohistochemical staining was performed with a well characterised monoclonal antibody to SNCA (LB 509; Zymed, San Francisco, California, USA) in the frontal cortex, cingulate gyrus, amygdala (including transentorhinal cortex), substantia nigra, locus coeruleus and medulla (level of the dorsal motor nucleus of the vagus), as previously described.22 Immunostained sections were assessed by a blinded (to clinical history) investigator (JBL) for the presence and severity of SNCA immunopositive intraneuronal, cytoplasmic inclusions, and neurites In each region, the SNCA pathological severity was ranked as absent (0), mild (1), moderate (2) or severe (3). Each case was classified for overall SNCA pathology using a modification of published criteria for Lewy body pathology.23
Zygosity
Monozygosity was confirmed by PCR amplification of polymorphic loci using primers labelled with fluorescent probes. The 28 markers were used from the ABI Prism Linkage mapping set, version 2.5, DH5 (http://home.appliedbiosystmes.com/).
PS1 genotyping
The PS1 gene was sequenced by DNA sequencing of exons 3–12 in genomic DNA using standard methods with a CEQ8000 (Beckman Coulter, Fullerton, California, USA).24
APOE genotype
APOE genotype was obtained from blood samples. Genotyping was performed using the dot blot method and replicated using a restriction enzyme digest method.25,26 Both methods yielded the same APOE genotype in all cases.
Results
Twin set A
The A twins were born in Minnesota and grew up in North Dakota where they both worked on farms (table 1). As adults, they both moved to the same small city in an agricultural region of Central Washington State where they remained until death. For the majority of their adult lives, they both worked in the same orchard. Neither smoked or drank alcohol. Both twins attended high school; A2 graduated but A1 did not.
Table 1 Clinical findings for three monozygotic twin pairs affected with Alzheimer's disease.
| A1 | A2 | B1* | B2 | C1 | C2 | |
|---|---|---|---|---|---|---|
| Sex | M | M | M | M | M | M |
| Age of onset (y) | 63 | 81 | 59 | 63 | 69 | 62 |
| Age at death (y) | 80 | 91 | 70 | 71 | 70 | 69 |
| Inter‐twin difference in age of onset (y) | 18 | 4 | 7 | |||
| Duration of disease (y) | 17 | 10 | 11 | 8 | 1 | 7 |
| APOE | ε3\ε4 | ε3\ε4 | ε3\ε3 | ε3\ε3 | ε3\ε4 | ε3\ε4 |
| Family history | − | + | + | |||
| Environment—child | Rural area | Small town, rural area | Small city, urban area | |||
| Environment—adult | Small city, rural area | Small city, rural area | Small town, urban area | |||
| Education | High school | High school diploma | High school diploma | High school diploma | High school diploma | High school diploma |
| Occupation | Orchardist | Orchardist | Truck driver | Manual labourer, then security guard | Sales man | Parts man |
| Cigarette use | U | No | No | No | No | No |
| Alcohol use | U | No | No | No | No | No |
| Early memory complaints | + | + | + | + | + | + |
| Hallucinations | − | − | − | − | − | + |
| Late onset increased tone | + | − | + | − | − | + |
| Late gait disturbance | − | − | + | − | − | + |
| Behavioural disturbance | + | − | − | − | − | + |
| Fluctuating disorientation | − | − | + | − | − | + |
| Antipsychotic use | + | − | − | − | − | − |
−, absent, +, present; U, unknown.
*Presenilin 1A79V mutation.
The twins were discordant for age of onset of AD and duration of disease. A1 developed dementia at age 63 years (age of death 80 years, disease duration 17 years). A2 developed dementia at age 81 years (age of death 91 years, disease duration 10 years). Both twins were APOE ε3/ε ε4. The family history was significant for one brother (age at death 74 years) out of five siblings being affected with mild forgetfulness.
Both A twins presented with progressive memory problems and the only symptomatic difference between them was the development of behavioural problems in A1 that required treatment with an antipsychotic medication. A1 also had a seizure disorder treated with phenytoin, and borderline diabetes, while A2 had a benign tremor. There was no documentation of parkinsonism in either twin.
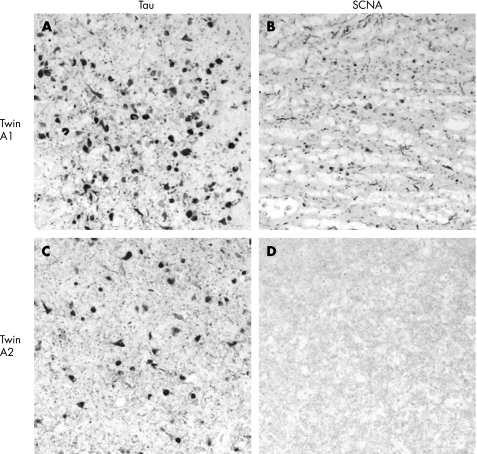
On neuropathological examination (table 2), the twins were concordant for AD pathological change with frequent NP in the neocortex21 and Braak stage VI NFT pathological change (table 2).20 They were discordant for LRP, with A1 having brainstem, limbic system and mild neocortical involvement, and A2 being negative for any LRP (fig 1, table 2). A1 had the earlier age of disease onset, longer duration of disease and more problematic behaviour. Neither twin had atheroma in the circle of Willis, and amyloid angiopathy was moderate in A1 and absent in A2.
Table 2 Neuropathological findings for three monozygotic twin pairs affected with Alzheimer's disease.
| A1 | A2 | B1 | B2 | C1 | C2 | |
|---|---|---|---|---|---|---|
| Atrophy | U | U | Mild frontal | Moderate–severe fronto‐temporal | Minimal frontal | Mild frontal |
| Brain weight (g) | U | U | 1140 | 1115 | 1211 | 1180 |
| Atheroma (circle of Willis) | − | − | + | + | − | − |
| Braak stage | VI | VI | V | V | V | V |
| CERAD NP | Frequent | Frequent | Frequent | Frequent | Frequent | Frequent |
| SNCA overall | Limbic | Negative | Neocortical | Limbic | Negative | Neocortical |
| SNCA amygdala | 2 | 0 | 3 | 3 | 0 | 3 |
| SNCA medulla | 0 | 0 | 3 | 0 | 0 | 3 |
| SNCA substantia nigra | 1 | 0 | 3 | 2 | 0 | 2 |
| SNCA locus coeruleus | u | 0 | 1 | 1 | 0 | 1 |
| SNCA transentorhinal cortex | 0 | 0 | 3 | 1 | 0 | 3 |
| SNCA cingulate gyrus | 1 | 0 | 3 | 2 | 0 | 2 |
| SNCA Frontal cortex | 1 | 0 | 2 | 0 | 0 | 2 |
| Cerebral amyloid angiopathy | Mild–moderate | None | Mild | Mild (moderate in occipital lobe) | Moderate–severe | Moderate–severe |
+, inclusions present; −, inclusions absent; NFT, neurofibrillary tangles; SNCA, alpha synuclein; U, unknown.
Semiquantitative SCNA pathology ratings: 0 = absent (no Lewy bodies), 1 = mild, 2 = moderate, 3 = severe.
Consortium to Establish a Registry for Alzheimer's Disease (CERAD) NP = frequent neuritic plaques.21
Braak stage V = widespread NFT involvement of the neocortex sparing or minimally involving the primary motor field, primary sensory areas and the unimodal secondary fields; VI = NFT in all neocortical regions.20
Figure 1 Amygdala, tau (PHF‐1 antibody, (A) and (C)) and alpha‐synuclein (LB509 (B) and (D)) immunostaining in the A twin brothers (twin A1 (A) and (B); twin A2 (C) and (D)). While both brothers had tau pathology, only one (twin A1) had additional alpha‐synuclein (SNCA) immunopositive inclusions and neurites.
Twin set B
The B twins grew up in a farming community in Northeastern Washington State and as teenagers worked in orchards (table 1). In mid‐life, B1 moved to a small city in an agricultural region of Southeastern Washington, while B2 moved to a small town in an urban region of the Puget Sound Area of Washington State in his 60s. B1 worked as a truck driver and B2 worked as a manual labourer and then as a security guard. Neither smoked or drank alcohol, and both graduated from high school.
Dementia developed in both MZ twins within 4 years of each other. B1 developed AD at the age of 59 years (age at death 70 years, duration of disease 11 years) and B2 at the age of 63 years (age at death 71 years, duration of disease 8 years). Both twins were APOE ε3/ε3. Because of the relatively early onset in the twins and other family members, they were screened for mutations in PS1. Several family members were found to have an A79V mutation in PS1.
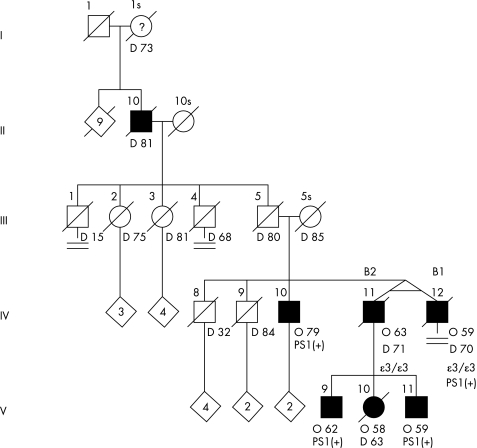
There was a strong family history of AD, including paternal great‐grandmother (age of death 73 years), paternal grandfather (age of death 81 years) and three children of B2 (child 1, age of onset 58 years, age of death 63 years; child 2, age of onset 62 years; and child 3, age of onset 59 years) (fig 2). Remarkably, a brother (IV‐10) with the PS1 mutation is living at age 83 years with moderate dementia (Mini‐Mental State Examination 15/30, Clinical Dementia Rating 1.0) and onset of memory loss at age 79 years.
Figure 2 Pedigree of twin pair B. O, Unaffected female; •, affected female, deceased; ▪, affected male. O, age at onset (years), D, age at death (years), ε, APOε allele, PS1(+), presenilin 1 mutation (A79V).
The clinical presentation of both B twins was similar, with dementia that was initially characterised by progressive memory impairment, confusion and disorientation. B1 had a history of possible fluctuating disorientation early in the course of his illness and developed a gait disturbance (slow, unsteady) late in the course of his illness while B2 did not. There was no other reported significant neurological history included.
Clinical information was available on the two affected sons of B2. Both sons had predominantly memory problems with one son developing behavioural disturbance with aggression, while the other did not. Neither son had a gait disturbance.
The twins were concordant for AD pathological change with frequent NP21 in the neocortex and Braak stage V NFT pathological change.20 They were discordant for severity of LRP (table 2) with B1 having more anatomically diffuse LRP (brainstem, limbic system and neocortex) than B2 (brainstem and limbic LRP without neocortical involvement). B1 had the earlier onset and longer duration of disease. Both twins had atheroma in the circle of Willis and evidence of recent cerebral infarcts at autopsy (B1 had a recent middle cerebral artery infarct while B2 had two microinfarts in his motor cortex and thalamus). B1 had less severe amyloid angiopathy than B2.
Twin set C
The C MZ twins lived and died in an urban area in the Puget Sound region of Western Washington State (table 1). C1 worked as a fireplace sales man and C2 worked as an automotive parts man. Neither smoked or drank alcohol. Both twins graduated from high school, and C1 spent one semester in college.
LOAD developed within 7 years of each other (C1 age of onset 69 years, age at death 70 years from metastatic adenocarcinoma, duration of disease 1 year; C2 age of onset 62 years, age at death 69 years, duration of disease 7 years). Both twins were APOE ε3/ε4.
The family history was notable for AD in at least four other family members (paternal grandmother (age of death 83 years), sister (age of onset 74 years, age of death 84 years, autopsy confirmed AD), brother (age of onset 89 years) and paternal cousin (age of death 62 years)).
The clinical presentations of the C MZ twins differed from each other. C1 developed memory complaints only 1 year before death from metastatic cancer and at death did not have symptoms or signs of parkinsonism. His past neurological history was significant for a small stroke in his 50s with a normal head CT scan. Presentation of C2 involved features of both AD and parkinsonism. His illness onset was characterised by prominent hallucinations, fluctuating progressive confusion and paranoia with gait instability and freezing, but no resting tremor, which was suggestive of a diagnosis of dementia with Lewy bodies.23 The past neurological history of C2 was significant for a mild stroke in his 30s, myocardial ischaemia (in his 50s) and trigeminal neuralgia. Neither twin had received antipsychotic medication.
The twins were concordant for AD pathological change with frequent NP in the neocortex21 and Braak stage V NFT pathological change (table 2).20 They were discordant for LRP (table 2), being absent in C1 and anatomically diffuse (brainstem, limbic system and neocortical LRP) in C2. C2 had the earlier age of onset and the longer disease duration. Neither of the twins had atheroma in the circle of Willis. Both twins and moderate to severe amyloid angiopathy and C2 had a small old inferior parietal haemorrhage.
Discussion
Detailed studies of twins can provide support for genetic versus environmental factors in AD. This study is the first to our knowledge to assess the concordance/discordance of NP, NFT and LRP in three sets of MZ twins (six individuals) including one twin pair positive for a PS1 mutation (twin pair B).
This study raises some interesting findings. All of the twins met criteria for definite AD with a history of dementia and neuropathological findings consistent with AD.27
The semiquantitative NP and NFT staging was concordant within all twin pairs despite wide variability in the age of onset, clinical presentation and duration of disease. This variability was most marked in the two twin pairs (A and C) without a PS1 mutation. This lack of correlation between neuropathology and disease duration supports the concept of early deposition of tau and amyloid and confirms an important role for genetic factors in the development of NP and NFT in AD.
In contrast, LRP was not concordant within the MZ twin pairs and there was a suggestion of a relationship between LRP and age of disease onset and/or duration of disease. The twin within each pair with the earlier age of onset and longer duration of disease had the greater LRP burden. This difference was most marked in the two twin pairs without the PS1 mutation. A similar relationship between duration of disease and severity of LRP has been reported in PS1 mutation associated familial AD.22
Recent studies have looked at the concordance of LRP in AD kindreds with PS1 and PS2 mutations22 and in kindreds with familial LOAD.28 The genetic influences for the development of LRP seem highest in families with the PS1 mutation compared with the PS2 mutations,22 while families with LOAD demonstrate more variability in the development of LRP, suggesting the influence of other factors.28 This pattern was reflected in our three twin pairs.
The discordance within the twin pairs for LRP suggests factors such as duration of disease, age of onset and environment have a greater impact than genetic factors on the development of LRP in AD. Of course, this may not be the case for LRP without coexistent AD.
Four of the six individuals had varying combinations of LRP and AD pathology. The presence of neocortical or limbic LRP tended to correlate with the presence of behavioural disturbances, fluctuating disorientation and gait disturbances, and the twin with the highest LRP burden fulfilled clinical criteria for dementia with Lewy bodies (C2).
The PS1 A79V mutation, observed in MZ twin pair B, has been reported by other investigators.29,30 Previously reported subjects with this mutation tended to have a later age of onset of disease (mid to late 50s) and a slower course than subjects with other PS1 mutations.29,30 This trend was true in our family B. To our knowledge, family member B IV‐10 represents the oldest age of onset (79 years) of a person with a PS1 mutation.
It is interesting that the twins reviewed in this study had such a wide range of age of onset (differences within pairs ranging from 4 to 18 years), most marked in the twins with LOAD rather than the PS1 twins. Other large studies have found the difference in age of onset to be smaller in MZ twins, between 0 and 7 years,9 although MZ twins can remain discordant in the development of AD for up to ∼21–22 years.10 The broad range of disease onset in MZ twins strongly implies the importance of environmental factors influencing this phenomenon. That these environmental factors remain largely unknown is underlined by the remarkably common environments experienced by the twins in this study.
Following submission of this paper, Kauwe and colleagues have reported a family with late onset AD and the A79V mutation in PS1 (Ann Neurol 2007;61:446–53).
Acknowledgements
We thank Lynn Greenup for technical assistance. Dr Bird is part of a licensing agreement with Athena Diagnostics, Inc.
Abbreviations
AD - Alzheimer's disease
EOAD - early onset Alzheimer's disease
LOAD - late onset Alzheimer's disease
LRP - Lewy related pathology
MZ - monozygotic
NFT - neurofibrillary tangles
NP - neuritic amyloid plaques
PS1 - presenilin 1
PS2 - presenilin 2
SNCA - alpha‐synuclein
Footnotes
Supported by NIA/NIH grant No P50 AG 005136‐22 and AG17586, Veterans Affairs research funds, and the Neurological Foundation of New Zealand.
Competing interests: None.
References
- 1.Holmes C. Genotype and phenotype in Alzheimer's disease. Br J Psychiatry 2002180131–134. [DOI] [PubMed] [Google Scholar]
- 2.Brickell K L, Steinbart E J, Rumbaugh M.et al Early onset Alzheimer's disease in families with late onset: an important subtype of Alzheimer's disease. Arch Neurol 2006631307–1311. [DOI] [PubMed] [Google Scholar]
- 3.Breitner J C, Jarvik G P, Plassman B L.et al Risk of Alzheimer disease with the epsilon4 allele for apolipoprotein E in a population‐based study of men aged 62–73 years. Alzheimer Dis Assoc Disord 19981240–44. [DOI] [PubMed] [Google Scholar]
- 4.Sherrington R, Rogaev E I, Liang Y.et al Cloning of a gene bearing missense mutations in early‐onset familial Alzheimer's disease. Nature 1995375754–760. [DOI] [PubMed] [Google Scholar]
- 5.Levy‐Lahad E, Wasco W, Poorkaj P.et al Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science 1995269973–977. [DOI] [PubMed] [Google Scholar]
- 6.Goate A, Chartier‐Harlin M C, Mullan M.et al Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 1991349704–706. [DOI] [PubMed] [Google Scholar]
- 7.Rogaeva E A, Fafel K C, Song Y Q.et al Screening for PS1 mutations in a referral‐based series of AD cases. 21 novel mutations. Neurology 200157621–625. [DOI] [PubMed] [Google Scholar]
- 8.Gatz M, Pedersen N L, Berg S.et al Heritability for Alzheimer's disease: the study of dementia in Swedish twins. J Gerontol A Biol Sci Med Sci 199752M117–M125. [DOI] [PubMed] [Google Scholar]
- 9.Gatz M, Reynolds C A, Fratiglioni L.et al Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry 200663168–174. [DOI] [PubMed] [Google Scholar]
- 10.Nee L E, Lippa C F. Alzheimer's disease in 22 twin pairs–13 year follow‐up: hormonal, infectious and traumatic factors. Dement Geriatr Cogn Disord 199910148–151. [DOI] [PubMed] [Google Scholar]
- 11.Gatz M, Mortimer J A, Fratiglioni L.et al Potentially modifiable risk factors for dementia in identical twins. Alzheimer Dement 20062110–117. [DOI] [PubMed] [Google Scholar]
- 12.Duda J E. Pathology and neurotransmitter abnormalities of dementia with Lewy bodies. Dement Geriatr Cogn Disord 2004173–14. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton R L. Lewy bodies in Alzheimer's disease: a neuropathological review of 145 cases using alpha‐synuclein immunohistochemistry. Brain Pathol 200010378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharman M G, Watt D C, Janota I.et al Case report: Alzheimer's disease in a mother and identical twin sons. Psychol Med 19799771–774. [DOI] [PubMed] [Google Scholar]
- 15.Cook R H, Schneck S A, Clark D B. Twins with Alzheimer's disease. Arch Neurol 198138300–301. [DOI] [PubMed] [Google Scholar]
- 16.Kilpatrick C, Burns R, Blumbergs P C. Identical twins with Alzheimer's disease. J Neurol Neurosurg Psychiatry 198346421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nee L E, Eldridge R, Sunderland T.et al Dementia of the Alzheimer type: clinical and family study of 22 twin pairs. Neurology 198737359–363. [DOI] [PubMed] [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M.et al Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 198434939–944. [DOI] [PubMed] [Google Scholar]
- 19.Bird T D, Levy‐Lahad E, Poorkaj P.et al Wide range in age of onset for chromosome 1 related familial Alzheimier's disease. Ann Neurol 199640932–936. [DOI] [PubMed] [Google Scholar]
- 20.Braak H, Braak E. Neuropathological staging of Alzheimer‐related changes. Acta Neuropathol 199182239–259. [DOI] [PubMed] [Google Scholar]
- 21.Mirra S S, Heyman A, McKeel D.et al The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 199141479–486. [DOI] [PubMed] [Google Scholar]
- 22.Leverenz J B, Fishel M A, Peskind E R.et al Lewy body pathology in familial Alzheimer's disease: evidence for disease and mutation‐specific pathologic phenotype. Arch Neurol 2006631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKeith I G, Dickson D W, Lowe J.et al Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005651863–1872. [DOI] [PubMed] [Google Scholar]
- 24.Kamimura K, Tanahashi H, Yamanaka H.et al Familial Alzheimer's disease genes in JNPanese. J Neurol Sci 199816076–81. [DOI] [PubMed] [Google Scholar]
- 25.Hixson J E, Vernier D T. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res 199031545–548. [PubMed] [Google Scholar]
- 26.Emi M, Wu L L, Robertson M A.et al Genotyping and sequence analysis of apolipoprotein E isoforms. Genomics 19883373–379. [DOI] [PubMed] [Google Scholar]
- 27.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging 199718(Suppl 4)S1–S2. [PubMed] [Google Scholar]
- 28.Tsuang D W, Riekse R G, Purganan K M.et al Lewy body pathology in late‐onset familial Alzheimer's disease: a clinicopathological case series. J Alzheimer Dis 20069235–242. [DOI] [PubMed] [Google Scholar]
- 29.Cruts M, van Duijn C M, Backhovens H.et al Estimation of the genetic contribution of presenilin‐1 and ‐2 mutations in a population‐based study of presenile Alzheimer disease. Hum Mol Genet 1998743–51. [DOI] [PubMed] [Google Scholar]
- 30.Finckh U, Muller‐Thomsen T, Mann U.et al High prevalence of pathogenic mutations in patients with early onset dementia detected by sequence analyses of four different genes. Am J Hum Genet 200066110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]