Ocular motor findings in Wernicke's encephalopathy (WE) include gaze evoked nystagmus (GEN), central positional nystagmus, weakness of abduction, internuclear ophthalmoplegia and horizontal or vertical gaze palsy to total ophthalmoplegia. Another feature of WE is vestibular paresis.1,2 Previous studies documented hypoactive vestibular responses to both caloric and rotational stimuli, and a short vestibulo‐ocular reflex (VOR) time constant. To address differential susceptibility of individual semicircular canals (SCC) according to stimulation frequency, we measured high acceleration VOR of the individual SCC using head impulse manoeuvres, and the low frequency VOR using bithermal caloric and rotatory chair tests in two patients with WE.
Case reports
Patient No 1
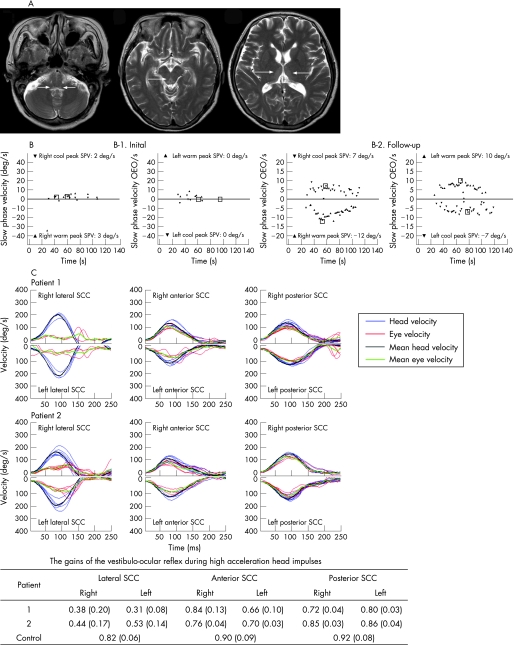
A 63‐year‐old woman had undergone Whipple's operation because of carcinoma of the ampulla of Vater 1 month previously and had received total parenteral nutrition (TPN). Two weeks after initiation of TPN, she began to suffer from anorexia, vomiting and vertigo which progressed to psychomotor slowing, apathy, forgetfulness and ataxia. On examination, she was awake but not attentive. She was fluent, but comprehension was impaired. The pupils were normal. Horizontal saccades were slowed and limited, and the limited ocular motor range did not improve with oculocephalic stimulation of the VOR. Other findings included horizontal GEN, limb dysmetria and severe truncal ataxia. T2 weighted MRIs showed hyperintense lesions at the periaqueductal gray matter, medial thalami and dorsal medulla (fig 1A). Three days after thiamine supplementation (100 mg intravenously daily), mental status, ataxia and horizontal gaze palsy began to improve.
Figure 1 (A) T2 weighted MRIs of patient No 1. Symmetrical hyperintense lesions are shown at dorsal portions of both the medulla, periaqueductal gray matter and medial portions of both thalami. (B) Bithermal caloric tests in patient No 1 show minimal responses in both ears initially (B‐1), which markedly improved 6 months after thiamine replacement (B‐2). (C) Head impulses in the plane of each semicircular canal (SCC) reveal severely impaired vestibulo‐ocular reflexes (VOR) from both lateral SCCs while the VORs from the vertical SCCs are normal or minimally impaired. Normal data were obtained from 10 healthy subjects (seven men and three women, aged 29–70 years, mean 52.1 (SD 13.6) years) without a history of vestibular or neurological disorders.
Patient No 2
A 40‐year‐old man with diabetes mellitus presented with a 4 day history of progressive vertigo, ataxia, apathy and psychomotor slowing. He had been a heavy drinker for several years. Examination showed decreased alertness, perceptual disturbance and impaired memory. Horizontal saccades were slow and limited to approximately 30° from the primary position in both directions and the ocular motor range did not improve with oculocephalic manoeuvres. He also showed horizontal GEN and bilateral limb dysmetria. He could not stand unaided. Brain MRI was normal. With parenteral thiamine supplementation, mental status, ocular signs and ataxia recovered rapidly. However, memory impairments and GEN remained for 2 weeks after symptom onset.
Oculography
Saccadic latency was increased to 322 ms in patient No 1 and to 289 ms in patient No 2 (normal 215 (17) ms). The horizontal saccades were hypometric with a mean accuracy of 71% in patient No 1 and 70% in patient No 2 (normal 93 (4)%). Horizontal saccades were slow with a mean velocity of 170°/s for 10° saccades in patient No 1 (normal >186°/s), whereas it was normal (193°/s) in patient No 2. In patient No 1, smooth pursuit was impaired with a gain of 0.4 for a peak target velocity of 10°/s and a frequency of 0.16 Hz (age matched normal 0.69 (0.11)). The gain of rightward smooth pursuit was 0.62 and that of leftward 0.49 in patient No 2 (normal 0.75 (0.07)).
Caloric test, rotatory chair test and pure tone audiometry
Patient No 1 showed no responses during bithermal caloric irrigation of either ear (fig 1B‐1). Sinusoidal rotation showed reduced gain and increased phase lead of the VOR. Time constants of pre‐ and post‐rotatory nystagmus were reduced significantly (3.7 s, normal 14.6 (3.7) s). In patient No 2, the caloric responses were slightly decreased, and the gain and phase of the VOR were normal while time constants of pre‐ and post‐rotatory nystagmus were moderately decreased (7.4 s). No patient showed hearing loss on pure tone audiometry.
Head impulse tests
In patient No 1, the VOR gain of each lateral SCC during head impulse was greatly reduced whereas gains of the vertical SCCs were minimally reduced for only left anterior and right posterior SCCs (fig 1C). In patient No 2, head impulse VOR gain of each lateral SCC was significantly reduced while that of vertical SCC was normal. Patient No 1 had a follow‐up evaluation 6 months later, and still showed memory dysfunction, GEN, subnormal gain and phase lead of the VOR during sinusoidal rotation, and decreased gain of the VOR during the head impulses in the lateral SCC planes even though the caloric responses improved markedly (fig 1B‐2). Time constants of the VOR increased slightly compared with the initial ones.
Discussion
Using the head impulse manoeuvre, we demonstrated differential susceptibility of the vestibular systems to thiamine deficiency. The VOR from the lateral SCCs was selectively or predominantly impaired and the vulnerability of the horizontal vestibular responses differed according to stimulation frequencies. According to previous histopathological studies, vestibular paresis in WE may be accounted for by lesions in the vestibular nucleus (VN).1 Neuropathological examinations of patients with WE have revealed lesions in the VN, especially in the medial VN (MVN), nucleus prepositus hypoglossi, nodulus and uvula. MVN was most vulnerable to thiamine deprivation,3 and histological abnormalities in the labyrinthine cristae and vestibular nerves were relatively minor in thiamine deficient pigeons.4
The vestibular neurons receiving different primary afferent input are distributed with some topographies across VN.5 The neurons activated by the saccule, utricle, and anterior and posterior canals are located mainly in the lateral (LVN) and descending vestibular nuclei, while the neurons activated by the lateral canal were found mainly in MVN and LVN. Accordingly, we suggest that different susceptibility of the vestibular systems in our patients may result from selective vulnerability of the neurons in the MVN.
On follow‐up evaluation, patient No 1 showed markedly improved caloric responses whereas the responses of rotatory chair and head impulse tests remained unchanged. In view of the selective deficit of high acceleration horizontal VOR during the acute phase in patient No 2, MVN neurons responsible for high acceleration horizontal VOR may be the most vulnerable to thiamine deficiency. A plausible explanation for this selective susceptibility may be high metabolic demands of the neurons responsible for the high acceleration VOR.
Alternatively, lesions of the abducens nucleus may impair conjugate horizontal saccadic, pursuit and vestibular eye movements in WE. However, no restriction in the horizontal ocular motor range, and selective deficit of high acceleration horizontal VOR and smooth pursuit in the presence of preserved saccadic velocities in patient No 2, do not support this assumption.
Footnotes
Ji Soo Kim was supported by the second stage Brain Korea 21 Project in 2006.
References
- 1.Furman J M, Becker J T. Vestibular responses in Wernicke's encephalopathy. Ann Neurol 198926669–674. [DOI] [PubMed] [Google Scholar]
- 2.Ghez C. Vestibular paresis: a clinical feature of Wernicke's disease. J Neurol Neurosurg Psychiatry 196932134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witt E D, Goldmann‐Rakic P S. Intermittent thiamine deficiency in the rhesus monkey. I. Progression of neurological signs and neuroanatomical lesions. Ann Neurol 198313376–395. [DOI] [PubMed] [Google Scholar]
- 4.Dreyfus P M, Victor M. Effects of thiamine deficiency on the central nervous system. Am J Clin Nutr 19619414–425. [DOI] [PubMed] [Google Scholar]
- 5.Brettler S C, Baker J F. Directional sensitivity of anterior, posterior, and horizontal canal vestibulo‐ocular neurons in the cat. Exp Brain Res 2001140432–442. [DOI] [PubMed] [Google Scholar]