Gastrointestinal symptoms in neurofibromatosis type 1 (NF1) is increasingly recognised, usually associated with gastrointestinal stromal tumours (GIST). We describe a patient with NF1 who presented with recurrent bowel symptoms and pseudo‐obstruction. At laparotomy, a segment of distended transverse colon was resected and histological examination revealed extensive dysplasia of the enteric nervous system different from GIST. These findings indicate that diffuse abnormalities of the gastrointestinal nerves may be symptomatic in patients with NF1 and should be considered as a possible cause of “functional bowel problems” in these patients. We suggest that the autonomic nervous system (eg, in the gastrointestinal system) can be involved similar to other peripheral nerves in NF1.
The patient, aged 73 years, had a NF1 diagnosed according to established consensus criteria.1 She had multiple small cutaneous neurofibromata, axillary freckling and café au lait spots. One large neurofibroma was removed at age 12 years. There was no evidence of skeletal abnormalities, optic gliomas, plexiform neurofibromata or malignant peripheral nerve sheath tumours. There was no family history of neurofibromata. Neurological tests, including examination of ability to sweat, was normal except for slight right‐sided clumsiness and facial weakness, as a result of a previous stroke. Remarkably, some years ago she presented with a picture of intermittent abdominal pain, bloating and diarrhoea. CT and coloscopy showed a dilated segment of the distal transverse colon, in keeping with the diagnosis of chronic intestinal pseudo‐obstruction. Because of the severity of her symptoms, she was referred for surgery.
At laparotomy, she underwent a transverse colectomy with resection of 21 cm of grossly distended colon. Centrally, the colonic circumference measured 36 cm. This led to some symptomatic improvement. However, she remains with considerable bowels symptoms, including gastroparesis and neurogenic bowel incontinence, confirmed by manometry and pudendus nerve stimulation, showing bilateral pathology. Additionally, she suffered from urinary stress incontinence and reduced bladder capacity, as shown by urodynamic measurements. MRI of the lumbar spine was normal.
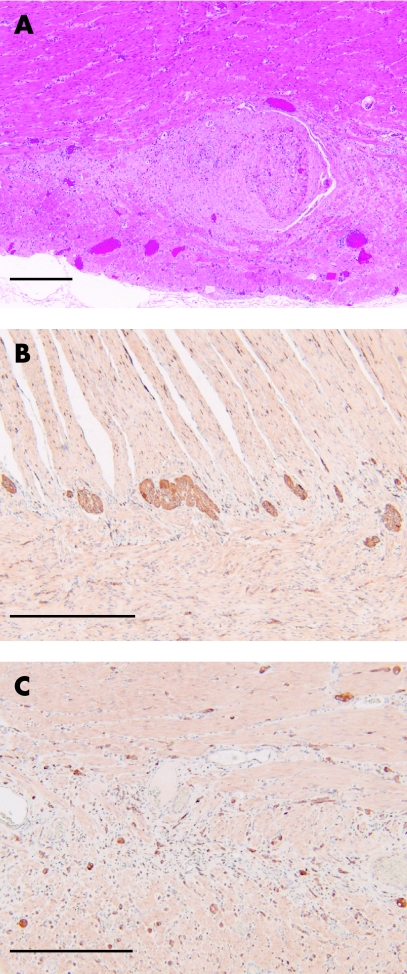
Pathological examination of the resected colon showed that in the mesentery and on the serosal surface there were small neurofibromata, measuring up to 0.5 cm in diameter and appearing largely fibrotic on histology. Sections from the resection ends did not show any significant histological abnormality. However, in much of the central area of the colon there was hyperplasia and tortuosity of the nerves, particularly within the myenteric plexus (fig 1A), and also to a lesser extent within the submucosa. These changes were multifocal and associated with Schwann cell proliferation. These areas of hyperplasia were CD34 negative. Within affected areas there was a loss of ganglion cells from the myenteric plexus and the presence of scattered neurons within the inner and outer muscle layers, some of which were binucleate (fig 1B, C). Ganglion cell hyperplasia was not seen in the areas sampled. There was patchy fibrosis and chronic inflammation of the muscularis layers, but no convincing muscle hyperplasia.
Figure 1 (A) Haematoxylin and eosin stained section showing hyperplastic nerves within the myenteric plexus. (B, C) Immunocytochemistry with an antibody to neuron specific enolase. (B) Ganglion cells can be seen distributed between the inner and outer layers of the muscularis externa at the resection margin. (C) Within the distended section of colon, there is loss of ganglion cells from the myenteric plexus, together with neurons scattered throughout the muscularis externa. Scale bar is 500 μm.
Gastrointestinal involvement in NF1 is increasingly recognised and its frequency is estimated at 11–25%.2 However, the vast majority of these cases are caused by GISTs,3 which usually occur in the small bowel and frequently present with gastrointestinal bleeding. Although GISTs, as well as ganglioneuromas, are reported in NF1, they were not present in our case, and the areas of hyperplasia were CD 34 negative (in contrast with GISTs). Neurofibromata may also rarely occur in the gastrointestinal nerves but are usually asymptomatic; those present in our case were unlikely to be sufficient to cause the symptoms. The histopathological features in our case most closely resembled those described by Fuller and Williams3 and Feinstat and colleagues,4 although the focal ganglion cell hyperplasia in the submucosa was not noted in our case, and are best described as neuronal intestinal dysplasia. Similar lesions have also been described in cases of multiple endocrine neoplasia, type 2b,5 indicating that separate genetic pathways can lead to this condition.
The persistent symptoms and electrophysiological abnormalities in our patient suggests more widespread involvement of the enteric nervous system. We suggest that diffuse abnormalities of the gastrointestinal autonomic nervous system may occur, in a similar way to other peripheral nerves,6 in NF1, and this possibility should be considered when patients present with what appears to be “functional bowel problems”.
Footnotes
Competing interests: None.
References
- 1.Gutmann D H, Aylsworth A, Carey J C.et al The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA 199727851–57. [PubMed] [Google Scholar]
- 2.Pinsk I, Dukhno O, Ovnat A.et al Gastrointestinal complications of von Recklinghausen's disease: two case reports and a review of the literature. Scand J Gastroenterol 2003381275–1278. [DOI] [PubMed] [Google Scholar]
- 3.Fuller C E, Williams G T. Gastrointestinal manifestations of type 1 neurofibromatosis (von Recklinghausen's disease). Histopathology 1991191–11. [DOI] [PubMed] [Google Scholar]
- 4.Feinstat T, Tesluk H, Schuffler M D.et al Megacolon and neurofibromatosis: a neuronal intestinal dysplasia. Case report and review of the literature. Gastroenterology 1984861573–1579. [PubMed] [Google Scholar]
- 5.Carney J A, Go V L, Sizemore G W.et al Alimentary‐tract ganglioneuromatosis. A major component of the syndrome of multiple endocrine neoplasia, type 2b. N Engl J Med 19762951287–1291. [DOI] [PubMed] [Google Scholar]
- 6.Ferner R E, Hughes R A, Hall S M.et al Neurofibromatous neuropathy in neurofibromatosis 1 (NF1). J Med Genet 200441837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]