Abstract
Background
Patients suffering from Parkinson's disease (PD) describe painful sensations that could be related to neuropathic pain. Experimental data have indicated the involvement of basal ganglia and dopaminergic pathways in central nociceptive processing.
Aim
The objective of this study was to assess and compare the effect of levodopa on the objective pain threshold in patients with PD and healthy subjects.
Methods
The objective pain threshold was assessed by the nociceptive flexion reflex (RIII) in 13 PD patients and 10 healthy subjects. Patients and healthy subjects were evaluated under two randomised conditions: with levodopa (ON) and without (OFF).
Results
Levodopa significantly increased the RIII threshold of PD patients (6.9 (1.2) mA in the OFF condition vs 8 (1.1) mA in the ON position; p = 0.02). RIII threshold was significantly lower in PD patients than in healthy subjects in the OFF condition (6.9 (1.2) mA vs 9.7 (3.4) mA; p = 0.02). RIII threshold did not change after levodopa administration in healthy subjects.
Conclusion
These results provide evidence of a dopaminergic modulation of objective pain threshold in PD patients. In addition, the decrease in RIII threshold in PD patients, in the OFF condition, compared with controls, confirms the existence of an objective pain perception disturbance in PD.
Pain is recognised as a feature of Parkinson's disease (PD) and is reported by 40–75% of patients with PD.1 Painful sensations are various (musculoskeletal, neuropathic pain) and may be present at any stage of the disease.2,3
Several anatomical, electrophysiological and pharmacological arguments are in favour of the involvement of the basal ganglia and dopaminergic pathways in central nociceptive processing.4
There are only a few controversial studies on pain perception in PD. The pain threshold of patients with PD has been found to be lower than, higher than or equal to that of healthy subjects.5,6,7 Recently, Djaldetti et al have shown that patients with PD have a lower subjective heat pain threshold than healthy subjects.8 To our knowledge, the effect of levodopa has only been assessed on the subjective pain threshold. Using two different experimental thermal stimulations (cold pressor test and thermotest with Peltier effect), we have previously shown that acute administration of levodopa significantly raised the subjective pain threshold of patients with PD.9,10
The nociceptive flexion reflex (RIII), a polysynaptic reflex, has been described as a useful tool for objectively investigating the pain threshold and its pharmacological modulation by analgesic drugs in normal subjects.11,12
The primary aim of the present study was to compare the effects of levodopa on objective pain threshold (RIII reflex) in pain free patients with PD and in healthy subjects. A secondary objective was to compare such parameters between these two groups.
Methods
Subjects
Thirteen patients with idiopathic PD without pain (four women and nine men) and 10 age matched healthy subjects (four women and six men) were included in the study. Patients were recruited from the Neuroscience Department, Toulouse University Hospital, Toulouse, France. They were included if they suffered from idiopathic PD according to the UKPDSBB criteria13 and had a positive response to long term levodopa treatment (improvement in the Unified Parkinson's Disease Rating Scale (UPDRS) (part III) ⩾50%). None suffered from acute or chronic pain related to PD (according to Ford's classification)3 or from any other disease (eg, osteoarthritis). None had Raynaud's phenomenon or peripheral neuropathy. Patients with cognitive defects (Mini‐Mental State Examination score below 25/30), or taking analgesic or antidepressant treatment, were excluded. All PD patients were taking antiparkinsonian dopaminergic drugs (levodopa and/or dopamine agonists).
Healthy subjects were recruited from the Toulouse Clinical Investigation Centre. Exclusion criteria were contraindication for levodopa or domperidone treatment, Raynaud's phenomenon, pregnancy and any analgesic or antidepressant treatment.
The protocol was approved by the local ethics committees (Toulouse I) and all subjects gave their written informed consent prior to the study.
Experimental protocol
PD patients and healthy subjects were evaluated under two different conditions: “OFF” (12 h after discontinuation of usual levodopa treatment in PD patients and without any treatment in healthy subjects) and “ON” (from 60 to 90 minutes after an acute oral levodopa challenge equivalent to the first morning dose plus 100 mg in PD patients, or 200 mg of levodopa in the healthy subjects). ON status was determined both from an improvement of at least 50% in the UPDRS (part III) and from the patient's opinion. The order of ON and OFF assessments was randomised by the staff of the Clinical Investigation Centre. Both groups were given domperidone treatment (60 mg/day) 3 days before sessions to reduce gastrointestinal side effects.
RIII nociceptive flexion reflex
We recorded nociceptive reflexes using a previously described methodology11,14 with an Oxford synergy data acquisition electromyography device.
The subjects were seated comfortably and were in complete muscular relaxation. The right sural nerve was stimulated by a pair of surface electrodes placed in the retro‐malleolar patch. The electrical stimulation consisted of a train of five rectangular pulses (each of 1 ms duration), delivered over 21 ms from a constant current stimulator (stimulation rate 0.2 Hz). Three series of 11 random intensities (6–16 mA) were applied. The electromyographic responses were recorded by a pair of surface electrodes placed over the ipsilateral biceps femoris muscle. EMG responses were amplified, digitised, full wave rectified and identified as a multiphasic signal and integrated within a time window from 90 to 180 ms after stimulus onset.
For each intensity of stimulation delivered (6–16 mA), the subject rated the sensation evoked by the electrical stimulation on a visual analogue scale in which 0 = “no pain” and 10 = “worst pain”.
Objective pain threshold (RIII threshold) was defined as the minimal intensity inducing a RIII reflex response (mean of the three measures).
Data analysis
In each group, between session differences were analysed using a non‐parametric match pairs Wilcoxon's rank sum test. Between group differences were analysed using an unpaired non‐parametric Mann–Whitney test. Statistical computing was performed with the Statview software, version 5. Statistical tests were considered significant at p<0.05. All values were expressed as mean (SD).
Results
Clinical characteristics
The age of patients with PD (61.5 (9.6) years, range 40–77) and healthy subjects (57.7 (4.4) years, range 51–63) were not significantly different. Mean duration of PD was 7.3 (5.3) years (range 1–19). In the ON condition, the levodopa dose was significantly lower in healthy subjects than in PD patients (200 vs 265 (90) mg, respectively; p = 0.03). In patients with PD, UPDRS part III was reduced by 50% in the ON condition compared with the OFF condition (10.8 (8.8) and 21.2 (11.8), respectively; p = 0.0015). No side effects were reported in the control group.
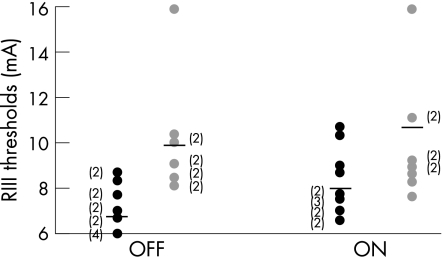
The RIII threshold (table 1, fig 1)
Table 1 Effect of levodopa on objective pain thresholds (RIII threshold) in patients with PD and in healthy subjects.
| RIII threshold | OFF condition | ON condition | p Value |
|---|---|---|---|
| PD patients | 6.9 (1.2) | 8 (1.1) | 0.02 |
| Healthy subjects | 9.7 (3.4) | 10.3 (3.4) | 0.4 |
| p Value | 0.02 | 0.1 |
PD, Parkinson's disease; RIII, nociceptive flexion reflex.
Values are mean (SD) mA.
Figure 1 Individual data for nociceptive flexion reflex (RIII) threshold in patients with Parkinson's disease (black circle) and in healthy subjects (grey circle) under the two conditions (OFF/ON). The horizontal black lines plot the mean of each group. (Number of subjects).
In PD patients, levodopa significantly increased the RIII threshold (OFF 6.9 (1.2) mA vs ON 8 (1.05) mA). In healthy subjects, the RIII threshold remained unchanged after levodopa. In the OFF condition, the RIII threshold was significantly lower in PD patients than in healthy subjects (6.9 (1.2) vs 9.7 (3.4) mA, respectively) whereas in the ON condition, the RIII threshold was no longer significantly different between the two groups.
In patients with PD, under the OFF and ON conditions, subjective pain threshold (11.3 (3) mA and 12.8 (3.1) mA, respectively) significantly correlated with RIII threshold (p = 0.001).
Discussion
This study demonstrates that levodopa significantly raises the objective pain threshold in pain‐free PD patients but not in healthy subjects. Moreover, patients with PD had a lower objective pain threshold than healthy subjects. To our knowledge, this is the first study reporting an effect of levodopa on the objective pain threshold in patients with PD.
Levodopa effect on RIII threshold in PD patients and in healthy subjects
In rodents, levodopa was reported to induce variable effects on pain, according to dosage.15 In patients with PD, the effect of dopaminergic drugs on painful sensations has been inconsistent.2,16 Nevertheless, as previously reported, levodopa had a significant effect on subjective pain threshold.9,10
Here we demonstrate in patients with PD that levodopa raised the objective pain threshold assessed by the RIII reflex threshold. A direct or indirect dopaminergic mechanism at the spinal level could be involved in this effect. Indeed, using the tail flick test in rats, some studies have shown that dopamine exerted antinociceptive effects through D2 subtype receptors at the spinal level.17 Apart from such spinal mechanisms, higher cerebral analgesic mechanisms may also be considered to explain this levodopa effect. We recently reported, using positron emission tomography (H215O) neuroimaging during experimental pain stimulation, that PD patients exhibited overactivation in several nociceptive cortical areas which were reduced by administration of levodopa.9
In agreement with our previous data, levodopa did not seem to have substantial effects in healthy subjects in this study.9 However, the dose of levodopa administered to healthy volunteers was smaller than those used in patients with PD and therefore we could not exclude the fact that a higher dose of levodopa would not produce a significant effect.
Comparisons of RIII threshold between PD patients and healthy subjects
RIII threshold was lower in our patients with PD than in healthy subjects. This observation supports the fact that patients with PD are likely to have abnormal pain thresholds, as suggested by previous studies,8,9 and in spite of different results reported by Guieu and colleagues7 that may be related to differences in electrical stimulation methods and the pharmacological status of patients with PD patients with regard to treatment.
From pharmacological, electrophysiological and imaging based findings it has been suggested that the basal ganglia could be involved in nociceptive processings.4 In rats, stimulation of the substantia nigra produces strong inhibition of the activation of the dorsal horns and thalamus parafascicular neurons elicited by noxious stimuli.18 Moreover, a fluorodopa positron emission tomography study has provided evidence of the involvement of the nigrostriatal dopaminergic system in patients suffering from chronic pain.19
In conclusion, despite a few methodological limits in our open study, we showed that acute administration of levodopa raised the objective pain threshold in patients with PD, and we confirmed the involvement of the dopaminergic system in central nociception in these patients. Further control studies, using placebo, should confirm these preliminary results in other groups of patients with painful PD.
Acknowledgements
The authors thank the staff of Toulouse Clinical Investigation Centre for patient management and Dr G Tap (biostatistics) for advice in statistical analysis of the data. This work was supported by the France Parkinson Association.
Abbreviations
PD - Parkinson's disease
RIII - nociceptive flexion reflex
UPDRS - Unified Parkinson's Disease Rating Scale
Footnotes
Competing interests: None.
References
- 1.Guiffrida R V F, Bogousslavsky J, Ghika J. Pain in Parkinson's disease. Rev Neurol (Paris) 2005161407–418. [DOI] [PubMed] [Google Scholar]
- 2.Quinn N P, Koller W C, Lang A E.et al Painful Parkinson's disease. Lancet 198611366–1369. [DOI] [PubMed] [Google Scholar]
- 3.Ford B. Pain in Parkinson disease. Clin Neurosci 1998563–72. [PubMed] [Google Scholar]
- 4.Chudler E H, Dong W K. The role of the basal ganglia in nociception and pain. Pain 1995603–38. [DOI] [PubMed] [Google Scholar]
- 5.Massetani R, Lucchetti R, Vignocchi G.et al Pain threshold and polysynaptic components of the blink reflex in Parkinson's disease. Funct Neurol 19894199–202. [PubMed] [Google Scholar]
- 6.Urakami K, Takahashi K, Matsushima E.et al The threshold of pain and neurotransmitter's change on pain in Parkinson's disease. Jpn J Psychiatry Neurol 199044589–593. [DOI] [PubMed] [Google Scholar]
- 7.Guieu R, Pouget J, Serratrice G. Nociceptive threshold and Parkinson disease. Rev Neurol (Paris) 1992148641–644. [PubMed] [Google Scholar]
- 8.Djaldetti R, Shifrin A, Rogowski Z.et al Quantitative measurement of pain sensation in patients with Parkinson disease. Neurology 2004622171–2175. [DOI] [PubMed] [Google Scholar]
- 9.Brefel‐Courbon C, Payoux P, Thalamas C.et al Effect of levodopa on pain threshold in Parkinson's disease: A clinical and positron emission tomography study. Mov Disord 2005201557–1563. [DOI] [PubMed] [Google Scholar]
- 10.Slaoui T, Gerdelat‐Mas A, Ory‐Magne F.et al Levodopa modifies pain thresholds in Parkinson's disease patients. Rev Neurol 200716366–71. [DOI] [PubMed] [Google Scholar]
- 11.Willer J C. Comparative study of perceived pain and nociceptive flexion reflex in man. Pain 1977369–80. [DOI] [PubMed] [Google Scholar]
- 12.Guirimand F, Dupont X, Bouhassira D.et al Nefopams strongly depresses the nociceptive flexion (R(III)) reflex in humans. Pain 199980399–404. [DOI] [PubMed] [Google Scholar]
- 13.Gibb W R, Lees A J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 198851745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hugon M. Exteroceptive reflexes to stimulation of the sural nerve in normal man. New Dev Electromyogr Clin Neurophysiol 19733713–729. [Google Scholar]
- 15.Paalzow G H. L‐dopa induces opposing effects on pain in intact rats: (−)‐sulpiride, SCH 23390 or alpha‐methyl‐DL‐p‐tyrosine methylester hydrochloride reveals profound hyperalgesia in large antinociceptive doses. J Pharmacol Exp Ther 1992263470–479. [PubMed] [Google Scholar]
- 16.Nutt J G, Carter J H. Sensory symptoms in parkinsonism related to central dopaminergic function. Lancet 19842456–457. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q S, Qiao J T, Dafny N. D2 dopamine receptor involvement in spinal dopamine‐produced antinociception. Life Sci 1992511485–1492. [DOI] [PubMed] [Google Scholar]
- 18.Barnes C D, Fung S J, Adams W L. Inhibitory effects of substantia nigra on impulse transmission from nociceptors. Pain 19796207–215. [DOI] [PubMed] [Google Scholar]
- 19.Jaaskelainen S K, Rinne J O, Forssell H.et al Role of the dopaminergic system in chronic pain—a fluorodopa‐PET study. Pain 200190257–260. [DOI] [PubMed] [Google Scholar]