Abstract
Blink reflexes (BR) to electric stimuli of the supraorbital nerve were recorded in 26 patients with dementia with Lewy bodies (DLB), 26 patients with multiple system atrophy, 26 patients with Parkinson's disease, with or without REM sleep behaviour disorder (RBD), and in 20 patients with Alzheimer's disease and 20 with progressive supranuclear palsy without RBD, and compared with recordings in 30 healthy controls. BR were significantly delayed (p<0.001) only in DLB patients in comparison with controls and with the other groups of patients; 14 (53.8%) patients had BR latency above 2 SD of the control mean, ranging from 36.1 to 46.3 ms. BR latency was not related to the presence of RBD, while a Spearman correlation rho of 0.68 was found for scores assessing the presence of cognitive fluctuations. R2 delay was prominently (71.5%) bilateral.
The electric blink reflex (BR) is a neurophysiological technique exploring pontine structures through a reflex arc connecting nuclei of the fifth to nuclei of the seventh cranial nerve.
It consists of three separate responses, R1, R2 and R3, the first one generated in the trigemino‐facial reflex arc, and the second and third ones generated in polysynaptic pathways involving the brainstem reticular formation.1 Clinically, the BR is used to evaluate brainstem lesions and it has been applied in clinical neurophysiological studies of brainstem lesions and neurodegenerative disorders.2,3,4
A recent pathophysiological hypothesis5 suggested that in dementia with Lewy bodies (DLB) and Parkinson's disease (PD), the brainstem is the site of initial lesions, consisting of α‐synuclein deposits. Synucleinopathy ascends from the brainstem, progressively involving the lower brainstem and inducing the appearance of REM sleep behaviour disorder (RBD), then the mesencephalus, inducing the occurrence of parkinsonism, and finally the limbic structures, inducing hallucinations and psychosis, and cortical areas, inducing cognitive disorders.
We hypothesise that BR might be altered in patients with DLB or other parkinsonisms presenting with RBD. To test this hypothesis, we recorded BR in 26 patients with DLB, 26 patients with multiple system atrophy (MSA), 26 patients with PD, with or without RBD, 20 patients with progressive supranuclear palsy (PSP) and 20 patients with Alzheimer's disease (AD) without RBD, and compared them with 30 age matched controls.
Material and methods
Subjects
From 2002 to 2006, 26 patients with DLB, 20 with AD, 26 with PD, 26 with MSA and 20 with PSP were randomly selected from 1400 patients admitted to our dementia register, which is part of the National CRONOS Registry of Dementia, and from 1056 patients admitted to our movement disorder clinic.
DLB and AD presented with progressive cognitive deterioration in the preceding 8–18 months.
Diagnosis of probable AD was based on the criteria of the National Institute of Neurological and Communication Disorders and Stroke‐Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA).6
Diagnosis of DLB was made according to consensus guidelines,7 MSA according to the consensus statement on the diagnosis of MSA,8 PSP according to international criteria9 and PD according to UK Brain Bank criteria.10
All the diagnoses were unchallenged after at least 2 years of follow‐up.
Patients underwent neuropsychological evaluations, including the Mini‐Mental State Examination, Unified Parkinson's Disease Rating Scale, Hoehn and Yahr scale and Cognitive Assessment of Fluctuations (CAF).11
Patient with DLB and AD were de novo patients and underwent neurophysiological evaluation before starting any pharmacological treatment.
The presence of RBD was evaluated according to minimal ICDS criteria12 and confirmed by polysomnography, according to methods previously described.13
Patients receiving concomitant medications, such as antidepressants, anticonvulsives, anticholinergics, typical or atypical antipsychotics or cholinesterase inhibitors, were not admitted to the study. Current treatment with L‐DOPA or dopamine agonists was allowed in MSA, PSP and PD patients. Thirty control subjects were recruited from our laboratory normative archive and matched with patients for age, gender and educational and occupational level. The resulting normative values overlapped with values reported previously.2
All subjects were right‐handed and had not consumed caffeine, nicotine or alcohol for at least 48 h before the clinical, neuropsychological and neurophysiological examinations. In MSA, PSP and PD patients, their morning treatments were withheld until neurophysiological assessments were completed. Patients (or their caregivers) and control subjects gave written informed consent to participate in the study.
The study was conducted according to the declaration of Helsinki and subsequent revision14 and was approved by our ethics committee.
Stimulation and recording
Subjects were seated comfortably in an armchair in a quiet room, with their eyes gently closed. The recordings took place in a temperature controlled room (approximately 25°C) in half light.
The cathode was placed over the supraorbital foramen and the anode 2 cm rostrally. Surface electrodes were placed on the inferior part of the orbicularis oculi muscles on each side, recording ipsilateral R1, and ipsilateral and contralateral R2 and R3. The ground electrode was placed under the chin.
Stimuli of 0.1 ms duration with intensity 5–10 mA elicited stable R1 in repeated trials. Because surface electrodes lay only a few centimetres away from the cathode, R1 tended to overlap the stimulus artefact, which could last more than 10 ms. A special amplifier with a short blocking time (0.1 ms) and low internal noise (0.5 ∝V at a bandwidth of 2 kHz) minimised the problem of stimulus artefact.
Signals were amplified and filtered (bandwidth 20–2000 Hz). We used interstimuli intervals of at least 7 s to avoid habituation, and 5–10 responses per site were elicited and stored for each patient or control.
Data analysis
The normality assumption of the distribution of BR values was tested separately for each group of patients (AD; DLB with and without RBD; PD with and without RBD; MSA with and without RBD; PSP and controls) using the Shapiro–Wilk statistic.
R1 and R2 components of the BR were recorded in all patients and were highly reproducible. Accordingly, each patient had six BR values in the analysis (ipsilateral R1, ipsilateral and contralateral R2 recorded after stimulation on the left and right side). First, R1 and R2 mean latency values were compared across groups separately for each stimulation side and recording, and then all R1 and all R2 latency values were merged and evaluated. Both analyses were carried out using ANOVA with Bonferroni correction and checked using the Kruskal–Wallis test. As both approaches gave similar results, only the complete R2 latency data (from both sides of the stimulation and recordings together) are presented to avoid redundancy.
All analyses were carried out using STATA, version 8.2 (Stata corp., College Station, Texas, USA). The examiner of the traces was blind to the diagnoses.
Results
Table 1 shows the disease characteristics and neurophysiological results, with patient groups divided into two subgroups depending on the presence of RBD.
Table 1 Disease characteristics and neurophysiological results in the patient groups.
| Variable | Controls | AD | PSP | PD RBD | PD no RBD | MSA RBD | MSA no RBD | DLB RBD | DLB no RBD |
|---|---|---|---|---|---|---|---|---|---|
| No of subjects | 30 | 20 | 20 | 7 | 19 | 4 | 22 | 17 | 9 |
| Age (y) | 71.3 (4.4) | 71.5 (4.4) | 69.2 (4.5) | 70.0 (4.0) | 69.9 (4.1) | 65.5 (3.9) | 64.8 (4.1) | 70.4 (4.9) | 69.7 (4.8) |
| Males (%) | 50 | 66.7 | 60 | 57.1 | 47.4 | 25 | 59.1 | 58.8 | 55.6 |
| MMSE | 28.9 (0.8) | 22.3 (1.3) | 27.5 (1.8) | 28.5 (1.3) | 28.6 (1.4) | 27.9 (1.7) | 28.1 (1.4) | 22.8 (1.5) | 23.2 (1.7) |
| ADAS‐cog | 12.0 (1.8) | 21.3 (6.4) | 12.4 (1.7) | 13.1 (1.5) | 12.9 (1.9) | 14.9 (2.9) | 13.5 (2.3) | 22.0 (5.5) | 21.7 (5.7) |
| CAF | – | 0 | 0 | 0 | 0 | 0 | 0 | 5.8 (2.0) | 5.9 (2.1) |
| UPDRS subscale III | – | 0.9 (1.0) | 11.2 (3.3) | 20.5 (7.4) | 19.5 (6.8) | 12.1 (3.8) | 12.3 (3.5) | 13.1 (6.6) | 13.1 (6.6) |
| Hoehn/Yahr stage | – | 0 | 3.0 (0.2) | 2.2 (0.3) | 2.1 (0.3) | 3.0 (0.2) | 3.0 (0.1) | 1.7 (0.3) | 1.7 (0.2) |
| Disease duration | – | 11.1 (3.0) | 10.1 (2.6) | 41.5 (10.5) | 41.2 (9.4) | 40.2 (9.2) | 41.0 (10.0) | 12.2 (3.1) | 12.3 (3.5) |
| R1 latency | 11.1 (0.4) | 11.3 (0.2) | 11.0 (0.1) | 11.2 (0.1) | 11.2 (0.2) | 11.4 (0.4) | 11.5 (0.4) | 11.2 (0.2) | 11.2 (0.1) |
| R2 latency | 31.1 (2.5) | 31.9 (2.9) | 31.9 (1.9) | 31.7 (1.7) | 31.6 (1.7) | 31.1 (0.8) | 31.5 (2.6) | 37.7 (5.0)*** | 38.0 (4.4)*** |
| R2 latency (95% CI) | 30.7–31.6 | 31.2–32.5 | 31.4–32.3 | 31.0–32.4 | 31.2–32.0 | 30.7–31.5 | 30.9–32.0 | 36.5–38.9 | 36.5–39.4 |
AD, Alzheimer's disease; CAF, Cognitive Assessment of Fluctuations; DLB, dementia with Lewy bodies; MMSE, Mini‐Mental State Examination; MSA, multiple system atrophy; PD, Parkinson's disease; PSP, progressive supranuclear palsy; RBD, REM sleep behaviour disorder; UPDRS, Unified Parkinson's Disease Rating Scale.
Unless stated otherwise, data are presented as mean (SD).
***Differences between these two groups and any other were statistically significant (p<0.001).
R1 latencies did not differentiate controls from the different groups of patients. R3 was scarcely reproducible in 32% of controls and 25–45% of the different patient groups, and therefore was not considered in statistical evaluations. R2 mean latencies recorded from AD, PD, MSA or PSP did not differ from control values or among the different groups of patients. R2 mean latencies recorded from DLB subjects were significantly delayed compared with controls and any other patient group (p<0.001 for every comparison).
In every group of patients the results were independent of the presence of RBD.
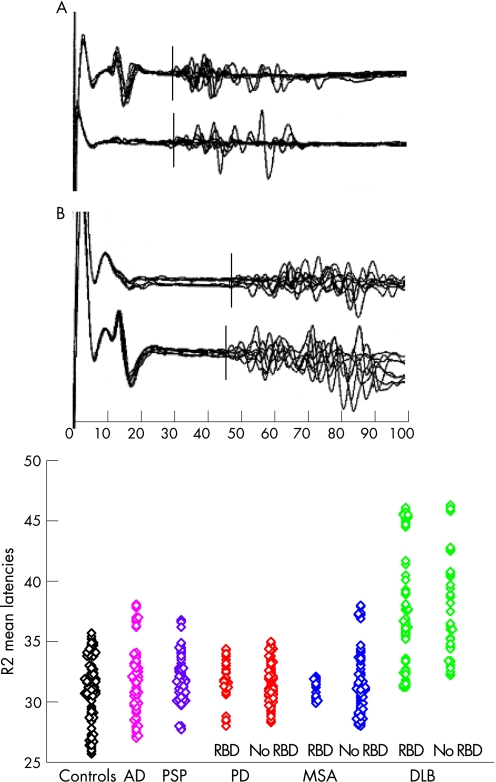
Figure 1 shows an example of a normal blink response in a control subject, a delayed R2 response in a patient affected by DLB without RBD, and a scatterplot of R2 latencies in the different groups of subjects.
Figure 1 Top: example of a blink response in a control subject (A) and in a patient with dementia with Lewy bodies (DLB) (B). Note the delayed R2 response in the DLB patient in both the ipsilateral and contralateral recordings. Bottom: Scatterplot of R2 mean latency in patients and controls. Patients with Parkinson's disease (PD), multiple system atrophy (MSA) and DLB are divided into two series. The first represents patients presenting with REM sleep behaviour disorder (RBD) and the second patients not experiencing RBD. For every subject, four R2 mean latency values are represented, resulting from both the right and left stimulations and ipsilateral and contralateral recordings.
R2 latencies from right‐sided or left‐sided stimulations overlapped (±0.1–0.3 ms) in all patients, but three patients affected by DLB had delayed R2 above 3 SD from the mean control value from stimulation of one side (two right, one left) while responses from the other side were 2 SD above the mean (two patients) and 1.5 SD above the mean (one patient). Ipsilateral and contralateral R2 had overlapping latencies in all patients and controls. Minor differences of 0.1–0.6 ms were observed in single responses and corrected by repeated stimuli. R2 latencies were above 3 SD in 10 DLB patients (38.5%) and above 2 SD in four more patients (total 53.8%). R2 latencies were above 2 SD in three patients with AD (15%), two with MSA (7.7%) and one with PSP (5%), and were below 2 SD in all PD patients. Delayed R2 were observed in all patients with CAF scores above 5, and latencies were related to CAF scores with Spearman rho = 0.68.
Discussion
BR recordings were previously described in MSA, PSP and PD patients. All reports showed R2 latencies inside the 2 SD of the mean, and only enhancement or inhibition of R1‐R2 was apparent in excitability–duration curve paradigms2,3,4 in untreated PD.
In all PD, MSA, PSP and AD patients, we found normal R1 and R2 latencies within 2 SD of the control mean, independent of the presence of RBD. We found R2 latencies clustering in the upper limits of normality or above the limits only in patients with DLB (fig 1). All findings were statistically significant.
Thus BR recordings may reveal brainstem dysfunction in DLB, but not in other parkinsonisms where different, yet definite, brainstem abnormalities are also described.
Abnormalities of another brainstem reflex, the auditory startle reaction, have been described as unspecific for DLB, being present in PD, PSP and MSA. However, DLB was the only parkinsonian disorder where a prolonged latency was found.15
According to the pathophysiological hypothesis,5 R2 latency delay may be attributed to the ascending synucleinopathy inducing the appearance of RBD. Our findings suggest that this possible correlation is controversial, as normal R2 latencies were observed in PD and MSA patients presenting with RBD, while delayed R2 latencies were recorded in five DLB patients who did not present with RBD (fig 1). Our findings suggest instead that the R2 latency delay in DLB is independent of the presence of RBD. A possible explanation is that the progressive caudo‐cranial involvement of different structures does not necessarily reflect cell loss and remains controversial as a pathophysiological model for PD progression.
The correlation with scores assessing cognitive fluctuations suggests that R2 abnormalities may indicate dysfunction of reticular brainstem pathways involved in vigilance regulation.
Current interpretations of BR neurophysiology1,16 suggest that R2 abnormalities should be ascribed to disruption of the afferent pathway when it is evident in both ipsilateral and contralateral responses to stimuli of any side, and efferent when the abnormality is observed in ipsilateral or contralateral responses of only one side, independent of the site of stimulation.
Only in three of the DLB patients presenting with R2 delays discrepant latencies on the two sides of stimulation were found, yet ipsilateral and contralateral responses always overlapped. Thus it is likely that the afferent pathway is prominently involved in DLB. An afferent delay of the R2 response has been described in patients with suprasegmental lesions.16 Thus dysfunction of brainstem reticular pathways might be present in DLB, but the R2 delay may also be explained by decreased corticoreticular drive secondary to spreading of Lewy bodies in polysynaptic pathways.
Abbreviations
AD - Alzheimer's disease
BR - blink reflexes
CAF - Cognitive Assessment of Fluctuations
DLB - dementia with Lewy bodies
MSA - multiple system atrophy
PD - Parkinson's disease
PSP - progressive supranuclear palsy
RBD - REM sleep behaviour disorder
Footnotes
Competing interests: None.
References
- 1.Cruccu G, Iannetti G D, Marx J J.et al Brainstem reflex circuits revisited. Brain 2005128386–394. [DOI] [PubMed] [Google Scholar]
- 2.Kimura J. Disorder of interneurons in parkinsonism. The orbicularis oculi reflex to paired stimuli. Brain 19739687–96. [DOI] [PubMed] [Google Scholar]
- 3.Valls‐Solé J, Valldeoriola F, Tolosa E.et al Distinctive abnormalities of facial reflexes in patients with progressive supranuclear palsy. Brain 19971201877–1883. [DOI] [PubMed] [Google Scholar]
- 4.Valls‐Solé J. Neurophysiological characterization of parkinsonian syndromes. Neurophysiol Clin 200030352–367. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Del Tredici K, Rub U.et al Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 200324197–211. [DOI] [PubMed] [Google Scholar]
- 6.McKhann G, Drachman D, Folstein M.et al Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer Disease. Neurology 198434393–394. [DOI] [PubMed] [Google Scholar]
- 7.Mc Keith I G, Dickson D W, Lowe J.et al Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 200565 pp 1863-72 erratum appears in 2005651992. [DOI] [PubMed] [Google Scholar]
- 8.Gilman S, Low P A, Quinn N.et al Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci 199916394–98. [DOI] [PubMed] [Google Scholar]
- 9.Litvan I, Agid Y, Calne D.et al Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele–Richardson–Olszewski syndrome): report of the NINDS‐SPSP international workshop. Neurology 1996471–9. [DOI] [PubMed] [Google Scholar]
- 10.Hughes A J, Daniel S E, Kilford L.et al Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinic‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 199255181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker M L, Ayre G A, Cummings J L.et al The Clinician Assessment of Fluctuation and the One Day Fluctuation Assessment Scale. Two methods to assess fluctuating confusion in dementia. Br J Psychiatry 2000177252–256. [DOI] [PubMed] [Google Scholar]
- 12.World Heath Organization The ICD‐10 classification of mental and behavioural disorders. Geneva: WHO, 1992
- 13.Onofrj M, Luciano A L, Iacono D.et al HLA typing does not predict REM sleep behaviour disorder and hallucinations in Parkinson's disease. Mov Disord 200318337–340. [DOI] [PubMed] [Google Scholar]
- 14.Declaration of Helsinki Recommendation Guiding Physicians in Biomedical Research involving human subjects. JAMA 1997277925–926. [PubMed] [Google Scholar]
- 15.Kofler M, Muller J, Wenning G K.et al The auditory startle reaction in Parkinsonian disorders. Mov Disord 20011662–71. [DOI] [PubMed] [Google Scholar]
- 16.Cruccu G, Deuschl G. The clinical use of brainstem reflexes and hand‐muscle reflexes. Clin Neurophysiol 2000111371–387. [DOI] [PubMed] [Google Scholar]