Abstract
Background
Progression rates in primary progressive multiple sclerosis (PPMS) vary widely and brain magnetisation transfer imaging (MTI) has potential as an early prognostic indicator. We investigated the predictive value of MTI and the longitudinal changes developing over 1 year in early PPMS.
Aims
To determine (1) whether baseline brain MTI parameters in early PPMS predict clinical changes over 1 year, independent of brain volume and (2) whether a change in magnetisation transfer (MT) parameters occurs over 1 year, independent of atrophy.
Methods
30 patients with PPMS within 5 years of symptom onset and 15 controls underwent MT and volumetric imaging studies, at baseline and at 1 year. Patients underwent clinical assessment using the Expanded Disability Status Scale (EDSS) and Multiple Sclerosis Functional Composite (MSFC), including the timed walk subtest (TWT). Normalised MT histograms were generated for whole brain, normal appearing brain tissue (NABT) and normal appearing white and grey matter (NAWM and NAGM) segments. Multiple regression analyses were performed to investigate whether baseline MTR parameters predicted clinical change over 1 year, adjusting for baseline brain volume. MTR changes over 1 year were assessed using paired t tests.
Results
In patients, lower baseline NAWM MTR predicted greater deterioration in EDSS and MSFC, particularly in walking ability measured by the TWT, independent of NAWM baseline volume (p = 0.001). NAGM MTR mean (p<0.001), and to a lesser extent NAWM mean (p = 0.011) and lesion MTR (p = 0.03), decreased over 1 year.
Conclusions
NAWM MTR may provide information on short term clinical prognosis in early PPMS. MTI is sensitive to brain tissue changes over 1 year in early PPMS, which were primarily seen in the NAGM.
Primary progressive multiple sclerosis (PPMS), in which disability develops in the absence of relapses, accounts for approximately 10% of all multiple sclerosis (MS) cases.1 The rate of deterioration varies widely,2 and at present little information on prognosis is available to patients and those planning their care.
The prognostic utility of conventional magnetic resonance imaging is limited by the relatively weak relationship between lesion load and disability.1 Instead, attention has focused on techniques examining damage to the normal appearing brain tissues (NABT), which has been implicated in the clinical evolution of PPMS.3 Magnetisation transfer imaging (MTI) has particular potential because, in addition to information about the whole brain, changes within the grey and white matter segments can be described. It measures magnetisation exchange between free water protons and protons bound to macromolecules, in the presence of an off‐resonance pulse saturating the bound protons only. The difference between this signal and that measured in the absence of preferential saturation is used to calculate the magnetisation transfer ratio (MTR). A reduced MTR is a putative marker for demyelination, and also reflects axonal loss.4,5 However, the exact relationship between MTR reduction and tissue atrophy is unclear, and measurement of MTR in patients with atrophy must take account of the potential of partial volume effects to reduce MTR.6
Previously, we have demonstrated a strong relationship between reduced MTR in the NABT and disability in early PPMS (within 5 years of onset).7 This early stage is known to be the most clinically dynamic phase of the condition,1 and therefore may be the most appropriate time to investigate longitudinal clinicoradiological correlations to find a useful prognostic indicator. We undertook the present study in a subgroup of this original cohort of patients with early PPMS, to establish whether baseline MTR variables could predict clinical changes over 1 year. We also investigated whether the MTR changed over 1 year, and how this related to the development of atrophy.
Methods
Subjects
Thirty‐one patients from an original cohort of 50, fulfilling the diagnostic criteria for definite or probable PPMS,8 within 5 years of symptom onset, underwent a magnetic resonance protocol at baseline and after 1 year. The mean and median separation of baseline and 1 year scans were both 12.3 months (range 10.1–15.2). One patient was later excluded because of a post‐processing failure (see below), leaving a total of 30 patients (17 males, 13 females, mean age 42.06 years (range 25–63)). Patient characteristics are given in table 1. Patients were recruited from clinics at the National Hospital for Neurology and Neurosurgery and other hospitals in Southeast England. Written and informed consent was obtained from all participants. The study was approved by the Joint Medical Ethics Committee of the National Hospital for Neurology and Neurosurgery and the Institute of Neurology, London, UK. Reasons for non‐attendance at 1 year in 15 patients were as follows: death, not MS related (n = 1), too overweight for the scanner (n = 1), scan missed because of scanner upgrade (n = 3), patient abroad (n = 1), moved away from London (n = 1), refusal to participate further in the study (n = 2), claustrophobia in the scanner (n = 1) and refused appointment (n = 5). In addition, four patients were scanned after the scanner upgrade, and excluded from this study. There was no significant difference in baseline Expanded Disability Status Scale (EDSS), Multiple Sclerosis Functional Composite (MSFC) or T2 lesion load between those patients studied at 1 year and those who were not.
Table 1 Patient characteristics.
| Sex (M/F) | 17/13 |
| Mean age (y) (range) | 42.06 (25–63) |
| Mean disease duration (y) (range) | 2.9 (1–5) |
| Presentation: cord/non‐cord symptoms | 23/7 |
| Median EDSS baseline/follow‐up (range) | 4.0 (1.5–7)/4.75 (2–7.5) p = 0.016 |
| Mean MSFC baseline/follow‐up (range) | 0.0533 (−2.67 to −1.07)/0.0161 (−2.88 to −0.96) p = 0.76 |
| Mean PASAT baseline/follow‐up (range) | 43.04 (0–60)/46.04 (3–60) p = 0.29 |
| Mean NHPT baseline/follow‐up (range) | 34.27 (17.13–96.23)/40.8991 (18.0–165.75) p = 0.218 |
| Mean TWT baseline/follow‐up (range) | 17.591 (3.65–180)/23.89 (3.70–180) p = 0.403 |
| Mean lesion load baseline (SD) | 11.46 (14.48) |
EDSS, Expanded Disability Status Scale; MSFC, Multiple Sclerosis Functional Composite; NHPT, nine hole peg test; PASAT, paced auditory serial addition test; TWT, timed walk test.
p values show the significance of the difference between baseline and follow‐up values, and were obtained from paired t tests and Wilcoxon rank tests.
Patients underwent neurological examination at baseline and at 1 year. They were scored on Kurtzke's EDSS,9 MSFC10 and its subtests (paced auditory serial addition test (PASAT), nine hole peg test (NHPT) and timed walk test (TWT)) were performed for the first time at baseline (without previous practice sessions) and on 22 of the patients at 1 year (12 males, 10 females, mean age 41.9 years (range 25–63)). There was no significant difference in baseline EDSS, MSFC or T2 lesion load, or in 1 year EDSS or T2 lesion load, between those patients scored for MSFC at 1 year and those who were not.
Fifteen healthy controls (nine males and six females, mean age 35.4 years (range 27–52)) were also scanned at baseline and at 1 year. The difference in age between patients and controls was adjusted for in the statistical analysis.
MRI acquisition
All scans were performed at baseline and after 1 year on a 1.5 Tesla GE Signa scanner (General Electric, Milwaukee, Illinois, USA). MTI was acquired using a two dimensional interleaved spin echo sequence described by Barker and colleagues11 (28 contiguous axial slices, repetition time 1720 ms, echo time 30/80 ms, number of excitations 0.75, acquired matrix 256×128, reconstructed matrix 256×256, field of view 240×240 mm), in which the saturated and unsaturated sequences are co‐registered and interleaved with simultaneously acquired proton density and T2 weighted images. A Hamming apodised three lobe sinc MT pulse (duration 16 ms, peak amplitude 23.2 μT, bandwidth 250 Hz, 1 kHz off water resonance) was applied, and the pixel MTR was calculated from the pre‐ (Mo) and post‐ (Ms) saturation signals using the formula [(Mo‐Ms)/Mo]×100 per cent units (pu).
All subjects also underwent a three dimensional inversion prepared fast spoiled gradient recall (3D FSPGR) sequence of the brain (124 contiguous axial slices, repetition time 13.3 ms, echo time 4.2 ms, inversion time 450 ms, matrix 256×160 (reconstructed matrix 256×256, final in plane resolution 1.17×1.17 mm), field of view 300×225 mm, slice thickness 1.5 mm) at baseline and after 1 year.
Image post processing
Magnetisation transfer imaging
Images were displayed on a Sun workstation (Sun Microsystems, Mountain View, California, USA) using DispImage software. Lesions were delineated with a semi‐automated contour thresholding technique (Plummer, Department of Medical Physics and Bioengineering, UCL, UK12) on the unsaturated PD images, with reference to the co‐registered T2 images, and used to create a lesion mask. The observer was blinded to the patients' clinical details. The T2 images underwent an automated segmentation procedure using SPM99 Software (Welcome Department of Cognitive Neurology, UK), producing non‐brain tissue, whole brain (WB), white matter and grey matter probability maps. We used SPM99 so that our results would be comparable with those from our cross sectional study.7 The probability maps were used to create WB, grey matter (GM) and white matter (WM) masks, which were then applied to the calculated MTR map in each subject to produce WB, GM and WM MTR maps. Segmentation of the MTR images was checked manually in all subjects. One patient's scans were incorrectly segmented, with WM accidentally included in the GM segment because of a very high lesion load. This patient was excluded from the analysis.
The T2 lesion masks were then applied to the WB, WM and GM MTR maps to produce normal appearing white matter (NAWM) and normal appearing grey matter (NAGM) maps. In the case of controls, where no lesions were found, segmentation produced normal WM and normal GM maps. To minimise partial volume voxels, we used a 10 pu threshold and eroded the outer and inner layer of voxels twice in the WM, and once in the GM (this is because the cortical GM was too thin to support more than one erosion).
MTR histograms were obtained for both the NAWM and NAGM in patients (or normal WM and normal GM in controls). To allow us to compare MTR histograms between subjects, the MTR histograms of each segment were normalised to the volume of that segment. There was a bin width of 0.1 pu and a smoothing window of 0.3 pu. MTR mean, peak height (PH) and peak location (PL) measures were taken from each individual histogram.
MTR from lesions was calculated by applying an inverse of the lesion mask to the PD MTR images, to produce lesion MTR maps, and normalised histograms generated as above.
Atrophy
At each time point, lesions were contoured on the individual FSPGR scans using the software described above, to create a T1 weighted lesion mask. The observer was blinded to the clinical details. FSPGR images were segmented into WM, GM and CSF using SPM99, and the volume of each segment calculated as described by Chard and colleagues.13 The lesion mask was then subtracted from the WB, WM and GM T1 images and separate NAWM, NAGM and lesion segments were obtained, with their volumes in millilitres. Volume estimations were made using a caudal cut‐off at the last slice containing cerebellum. The following parameters were calculated:
total intracranial volume (TIV) = lesion volume (LV) + normal appearing white matter volume (NAWMV) + normal appearing grey matter volume (NAGMV) + CSF volume;
brain parenchymal fraction (BPF) = (LV + NAWMV + NAGMV)/TIV;
normal appearing white matter fraction (NAWMF) = NAWMV/TIV;
normal appearing grey matter fraction (NAGMF) = NAGMV/TIV.
Statistical analysis
Analysis was carried out using SPSS 10.0 (Chicago, Illinois, USA). Statistical significance is reported at the 5% level. Significant values for correlation coefficients are reported without correction for multiple comparisons to avoid type II errors.14
Clinical data
In analysing the change in EDSS scores, a one step deterioration on the scale was defined as an increase of 1 if the baseline EDSS was less than or equal to 5 or an increase of 0.5 if it was greater than 5.15 This gives greater weight to change in more disabled patients, in whom deterioration is harder to detect on the EDSS scale, and these steps have been regarded as equivalent in clinical trials.16 Z scores (z) were derived for the MSFC subtests using our own baseline sample as reference, and used to calculate the MSFC. One patient at baseline and two at 1 year were too disabled to complete the TWT, and were scored with the maximum time allowed for the TWT (180 s), as described by Hoogervorst and colleagues.17
Baseline MTR predictors
To determine whether baseline MTR in patients predicted clinical change, multiple linear regression analyses were performed for each clinical test and subtest, and each MTR parameter in each segment. Clinical score at 1 year was the dependent variable, and clinical score at baseline and baseline MTR parameters were independent variables, so that any relationship identified between baseline MTR variables and change in clinical score would be independent of any relationship between baseline MTR variables and baseline clinical score. Models were adjusted for age, gender and baseline intrasegmental volume (ie, WB analysis was adjusted for baseline BPF, NAWM for NAWMF and NAGM for NAGMF). Where significant correlations were found between baseline MTR parameters and clinical change, the strength of the association was assessed using partial correlations adjusted for age, gender and intrasegmental volume.
To further investigate the utility of baseline MTR measures in predicting clinical change, patients were divided into two groups: those who stayed the same or improved on EDSS over 1 year and those who worsened. The MTR parameter that most strongly predicted EDSS changes was then chosen, and patients were divided into two groups depending on whether the value of this parameter was lower or higher than a cut‐off below the lowest value in controls. A 2×2 table was then constructed showing the number of patients with low and normal MTR against those who worsened, or who stayed the same or improved on EDSS. The sensitivity, specificity, positive and negative predictive values and overall accuracy of using low NAWM MTR mean to predict clinical worsening were calculated according to standard methods.18
MTR change over 1 year in patients and controls
To determine MTR change over 1 year, paired t tests were used to compare MTR at baseline and at 1 year within patient and control groups. Multiple linear regression was then used to compare changes in each segmental MTR parameter between patient and control groups, adjusting for age and gender.
Relationship between MTR and atrophy
Pearson's correlations were obtained to establish the relationship between MTR change and change in brain volume in each segment. GM damage was also related to WM changes,19,20 and therefore the correlation between NAGM MTR decrease and change in NAWMF was also examined.
Results
Clinical changes
Clinical changes over 1 year are summarised in table 2. Patients progressed clinically with worsening EDSS scores (p = 0.012). There was a non‐significant decrease in mean MSFC, with worsening of the TWT and NHPT scores. PASAT scores improved over 1 year.
Table 2 Baseline MTR parameters predict change in clinical parameters over 1 year.
| MTR parameters at baseline | Change in EDSS | Change in MSFC | Change in zPASAT | Change in zNHPT | Change in zTWT | |
|---|---|---|---|---|---|---|
| WB | Mean | 0.013 (−0.46) | 0.001 (0.67) | 0.034 (0.43) | 0.176 | <0.001 (0.71) |
| PH | 0.546 | 0.001 (0.64) | 0.032 (0.44) | 0.854 | 0.001 (0.64) | |
| PL | 0.073 (−0.34) | 0.006 (0.58) | 0.184 | 0.083 | <0.001 (0.70) | |
| NAWM | Mean | 0.036 (−0.39) | 0.001 (0.68) | 0.055 | 0.255 | <0.001 (0.81) |
| PH | 0.291 | <0.001 (0.54) | 0.101 | 0.825 | <0.001 (0.75) | |
| PL | 0.040 (−0.38) | 0.680 | 0.072 | 0.203 | 0.001 (0.64) | |
| NAGM | Mean | 0.648 | 0.108 | 0.336 | 0.600 | 0.058 (0.40) |
| PH | 0.724 | 0.084 | 0.565 | 0.965 | 0.007 (0.55) | |
| PL | 0.725 | 0.019 (0.51) | 0.523 | 0.190 | 0.004 (0.58) | |
| Lesions | Mean | 0.181 | 0.047 (0.44) | 0.052 (0.40) | 0.687 | 0.012 (0.52) |
| PH | 0.123 | 0.767 | 0.656 | 0.929 | 0.739 | |
| PL | 0.331 | 0.011 (0.54) | 0.013 (0.50) | 0.816 | 0.009 (0.53) | |
EDSS, Expanded Disability Status Scale; MSFC, Multiple Sclerosis Functional Composite; MTR, magnetisation transfer ratio; NAGM, normal appearing grey matter; NAWM, normal appearing white matter; PH, peak height; PL, peak location; WB, whole brain; zNHPT, z score for the nine hole peg test; zPASAT, z score for the paced auditory serial addition test; zTWT, z score for timed walk test.
Significant p values (p<0.05) shown in bold typeface, with r values in parentheses. p values were obtained from multiple linear regression analysis with age, gender and intrasegmental volume as covariates where significant. r values were obtained from partial correlation coefficients.
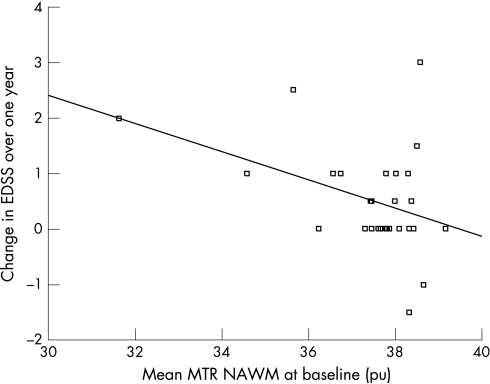
Predictive value of baseline MTR (tables 2 and 3, fig 1)
Table 3 Baseline NAWM MTR mean below 37 pu predicts worsening on EDSS over 1 year.
| NAWM MTR mean <37 pu | NAWM MTR mean ⩾37 pu | Total | ||
|---|---|---|---|---|
| Deterioration on EDSS | 5 | 5 | 10 | Sensitivity 50% |
| EDSS stable or improved | 1 | 19 | 20 | Specificity 95% |
| Total | 6 | 24 | 30 | |
| PPV 83% | NPV 79% | Overall accuracy 80% |
EDSS, Expanded Disability Status Scale; MSFC, Multiple Sclerosis Functional Composite; MTR, magnetisation transfer ratio; NAWM, normal appearing white matter; NPV, negative predictive value; PPV, positive predictive value; pu, per cent units; zPASAT, z score for the paced auditory serial addition test; zNHPT, z score for the nine hole peg test; zTWT, z score for timed walk test.
Figure 1 Baseline NAWM MTR mean predicts change in EDSS over 1 year. Note The positive correlation is maintained when data are analysed without the two outlying patients with EDSS change of +2 and +3, respectively (p = 0.027, r = −0.40). EDSS, Expanded Disability Status Scale; MTR, magnetisation transfer ratio; NAWM, normal appearing white matter; pu, per cent units.
Lower MTR at baseline predicted clinical progression with a greater increase in EDSS and a greater decrease in MSFC over 1 year after adjusting for age, gender and intrasegmental atrophy. Baseline WB MTR (mean p = 0.013) predicted change in EDSS. There was no contribution from lesion MTR, but NAWM MTR parameters predicted EDSS change significantly (mean p = 0.036 and PH p = 0.040). It was calculated that a baseline NAWM MTR mean below 37.0 pu (this was chosen as a cut‐off because it was well below the lowest MTR value in controls, which was 37.68) was able to predict worsening on the EDSS over 1 year with a specificity of 95%, sensitivity of 50%, positive predictive value of 83% and negative predictive value of 79% (table 3). The overall accuracy of the test, indicating the proportion of predictions which are correct, was 80%.
For the MSFC change, the most complete predictor was the WB MTR (mean and PH p = 0.001, PL p = 0.006). This prediction emerged largely from the NAWM segment (mean and PH p<0.001). The NAGM MTR (PL p = 0.019) and the lesion MTR (mean p = 0.047, PL p = 0.011) were weak predictors of MSFC change.
Of the MSFC subtests, change in the z score for the timed walk test (zTWT) was predicted by baseline NAWM (mean, PH, PL p<0.001) and NAGM parameters (PH p = 0.007, PL p = 0.004), and lesion MTR (mean p = 0.012, PL p = 0.009). Z score for the paced auditory serial addition test (zPASAT) was weakly predicted by WB (mean and PH) and by lesion MTR (PL p = 0.013). Although there was a group improvement in zPASAT, the positive correlation demonstrates that individuals with lower MTR values were more likely to have a lower zPASAT score after 1 year.
MTR changes over 1 year (table 4)
Table 4 MTR changes over 1 year in patients and controls.
| MTR parameter | Mean baseline MTR (SD) | Mean 1 year MTR (SD) | p Value | 95% CI | Change in patients vs controls (p value) | |
|---|---|---|---|---|---|---|
| Patients | ||||||
| WB | Mean | 33.5107 (1.4312) | 33.2006 (1.5693) | <0.001 | −44.5791 to −17.4339 | – |
| PH | 0.0099 (0.0013) | 0.0096 (0.0014) | 0.025 | −.0004 to −0.0001 | – | |
| PL | 36.5067 (1.2301) | 36.1300 (1.3742) | 0.014 | −1.8110 to −0.2263 | – | |
| NAWM | Mean | 37.4810 (1.4571) | 37.2447 (1.5674) | 0.011 | −41.5150 to −5.7583 | 0.497 |
| PH | 0.0187 (0.0027) | 0.0181 (0.0031) | 0.147 | −0.0013 to 0.0002 | 0.589 | |
| PL | 37.7733 (1.0299) | 37.5567 (1.1110) | 0.013 | −38.3395 to −4.9938 | 0.272 | |
| NAGM | Mean | 31.3081 (0.9827) | 31.0357 (1.0273) | <0.001 | −39.6549 to −14.8370 | 0.024 |
| PH | 0.0105 (0.0014) | 0.0104 (0.0016) | 0.496 | −0.0004 to 0.0002 | 0.366 | |
| PL | 32.6367 (0.8544) | 32.2400 (0.9077) | 0.001 | −61.8225 to −17.5108 | 0.101 | |
| Lesion | Mean | 31.3756 (2.2917) | 31.0493 (2.3773) | 0.030 | −61.5595 to −3.3299 | – |
| PH | 0.0107 (0.00254) | 0.0117 (0.00379) | 0.184 | −0.0005 to 0.0025 | – | |
| PL | 34.2267 (2.2841) | 33.7533 (2.2841) | 0.091 | −102.7525 to 8.0859 | – | |
| Controls | ||||||
| WB | Mean | 34.7239 (0.3795) | 34.7249 (0.6411) | 0.201 | −24.6627 to 24.8495 | – |
| PH | 0.01060 (0.0009) | 0.01062 (0.0010) | 0.362 | −0.0001 to 0.0002 | – | |
| PL | 37.4200 (0.4459) | 37.0933 (0.7805) | 0.364 | −70.4856 to 5.1522 | – | |
| NWM | Mean | 38.3962 (0.3963) | 38.2275 (0.5708) | 0.994 | −43.8716 to 10.1271 | – |
| PH | 0.0202 (0.0022) | 0.0199 (0.0023) | 0.412 | −0.0010 to 0.0004 | – | |
| PL | 38.5133 (0.3739) | 38.4000 (0.5278) | 0.085 | −37.2122 to 14.5455 | – | |
| NGM | Mean | 32.9007 (0.4440) | 32.2848 (0.6776) | 0.886 | −26.8588 to 23.4277 | – |
| PH | 0.01214 (0.0013) | 0.0122 (0.0015) | 0.494 | −0.0003 to 0.0006 | – | |
| PL | 33.1333 (0.5551) | 33.0867 (0.7434) | 0.734 | −33.5838 to 24.2504 | – | |
MTR, magnetisation transfer ratio; NAGM, normal appearing grey matter; NAWM, normal appearing white matter; NGM, normal grey matter; NWM, normal white matter; PH, peak height; PL, peak location; WB, whole brain.
Mean and peak location MTR values are in percentage units, with significant p values (p<0.05) shown in bold typeface. p values were derived from paired t tests for ingroup comparisons, and multiple linear regression adjusting for age and gender for between group comparisons.
In patients, there was a decrease in all MTR mean values at 1 year, with lesion MTR showing the least significant decrease (p = 0.03), followed by NAWM (p = 0.011). All MTR peak location values, except for lesion MTR, also decreased: WB (p = 0.014), NAWM (p = 0.013) and NAGM (p = 0.001). A significant decrease in MTR peak height was only observed in WB (p = 0.025). In controls, there were no significant longitudinal changes in MTR. When comparing MTR changes between patient and control groups, significant differences were found in NAGM MTR mean (p = 0.02).
Mean T2 lesion volume increased from 11.5 ml to 16.2 ml.
Correlations between MTR and volume changes (table 5)
Table 5 Correlation of change in MTR with change in intrasegmental volume over 1 year.
| Change in MTR parameter | Correlation with intrasegmental volume change | |||
|---|---|---|---|---|
| p Value | r Value | r2 Value | ||
| WB | M | 0.148 | 0.280 | 0.078 |
| PH | 0.011 | 0.474 | 0.225 | |
| PL | 0.097 | 0.320 | 0.102 | |
| NAWM | M | 0.026 | 0.419 | 0.176 |
| PH | 0.008** | 0.492 | 0.242 | |
| PL | 0.028 | 0.415 | 0.172 | |
| NAGM | M | 0.609 | 0.101 | 0.010 |
| PH | <0.001** | 0.670 | 0.449 | |
| PL | 0.210 | 0.244 | 0.060 | |
M, mean; MTR, magnetisation transfer ratio; NAGM, normal appearing grey matter; NAWM, normal appearing white matter; PH, peak height; PL, peak location; r, correlation coefficient for Pearson's test; WB, whole brain.
p values are derived from Pearson's test. Significant values at p<0.05 are shown in bold typeface and **p<0.01.
Correlations were found between decrease in WB, NAWM and NAGM MTR peak height and decrease in corresponding intrasegmental volume (p = 0.011, p = 0.008, p = 0.001, respectively). Change in NAWM MTR mean and peak location parameters also correlated significantly with the progression of atrophy in corresponding brain tissues (p = 0.013, p = 0.026 and p = 0.028, respectively). There was no significant correlation between the NAGM MTR decrease and change in NAWMF.
Discussion
MTR parameters predict clinical change
This study shows, for the first time, that brain MTI is a modest predictor of clinical evolution in PPMS, over a relatively short period. Importantly, predictions survived adjustment for intrasegmental volume, demonstrating that the predictive value of MT imaging is independent of atrophy effects on MTR. Over a short study period, clinical and imaging changes are necessarily small, however, and further work will be necessary to establish the role of MTI as a prognostic indicator.
Over 1 year, patients progressed clinically, as demonstrated by a significant worsening of EDSS scores. Although MSFC scores deteriorated, the change was not statistically significant. This is likely to be due to several factors. Firstly, MSFC data were not available on all patients. Secondly, although there was a decline in mean zTWT and z score for the nine hole peg test (zNHPT) scores, there was an improvement in zPASAT over 1 year, which is likely to be a result of practice effects and reduced anxiety as patients became accustomed to testing.21,22 Lastly, patients who were unable to complete the TWT at baseline could not demonstrate reducing mobility on the MSFC, whereas their EDSS increased. Nevertheless, the MSFC data have value in this analysis as it is preferable to EDSS for investigating group differences within a sample.23,24
NAWM MTR parameters appear to be driving the prediction of EDSS change, and to be contributing most to the prediction of MSFC change. Furthermore, we found that when a cut‐off value of 37 pu was used for NAWM MTR mean, 83% of patients with a low MTR progressed on the EDSS scale over 1 year while 79% of those with a normal MTR stayed the same or improved. The overall sensitivity of the test for identifying those who would progress was 50%, with a specificity of 95%, and using a higher cut‐off would have increased sensitivity, but decreased specificity. Further studies in a larger cohort would be necessary to validate the use of a specific MTR value to predict disability in individual cases.
Lesion MTR predicted change in MSFC but not EDSS. Recent work in other types of MS has highlighted change in average lesion MTR over a 1 year period as a predictor of disability development over 8 years,25 and we may find that the changes we have observed in mean lesion MTR during the study have a predictive value for clinical changes in the longer term. There was a weak prediction of MSFC change from the GM segment, and this is discussed further below. Of note, NAWM peak height did not significantly predict EDSS change because significance was lost when the model was adjusted for baseline brain volume.
Regarding MSFC subtests, changes in the zTWT subscore were most strongly predicted by imaging parameters, emphasising mobility as the prime indicator of progression in this cohort. This is consistent with the group's composition: 23 of the 30 patients presented with a cord syndrome.
MTR decreases significantly over 1 year
To our knowledge, only one previous study evaluated MTR in longitudinal follow‐up of PPMS26: no significant change in MTR parameters in lesions, WB or NABT was found in nine PPMS patients over 1 year. Median disease duration in this PPMS group was 8 years, with a range of 3–14 years.
In our patients, there was a longitudinal decrease in NAGM MTR mean and peak location, and the former was significantly different from changes in controls. The decrease in NAWM MTR mean and peak location was less pronounced, and did not reach significance compared with controls. The magnitude of grey matter changes in MS have drawn comment in previous work,27,28 including a 2 year follow‐up of early relapsing and remitting MS29 which also demonstrated relatively greater GM MTR decrease compared with WM. Clinically isolated syndromes (CIS) patients have shown equivalent reduction in GM and WM MTR.30 In addition, diffusion tensor imaging has demonstrated marked progression of NAGM changes in advanced PPMS (mean disease duration 10 years) over 1 year, in the absence of significant change in the NAWM.31
This relatively extensive decrease in NAGM compared with NAWM MTR could be due to several factors. Firstly, lesions within the cortex and deep GM, which are more numerous in MS than previously thought,32,33,34, cannot be reliably detected on conventional MRI scans even at high field strengths35 because of signal intensities that overlap the surrounding GM.36 Thus the NAGM contained an unknown number of lesions, while visible WM lesions were masked out of the analysis. Secondly, the MTR decrease may be measuring a more general cortical demyelination, thought to be particularly prevalent in progressive forms of MS, which is not associated with focal inflammation.37 Thirdly, MTR in the GM may have been reduced via partial volume effects. GM atrophy without significant WM atrophy is known to have developed in this cohort over 1 year,38 and as brain volume decreases, there is an increase in the number of outer voxels at the brain–CSF interface. The result is more voxels containing both brain tissue (particularly GM) and CSF, which therefore have a lower MTR. However, steps were taken to minimise this effect. Two outer voxel erosions were carried out for the WM, and one for the GM, discarding voxels with an MTR value below 10 pu. MTR histograms were then normalised for brain volume. Furthermore, to elucidate the relationship between atrophy development and MTR changes, we correlated the two measures. We found that although the significant MTR reductions occurred in the mean and peak location of the NAGM histogram, GM volume changes correlated best with change in NAGM peak height, which had not reduced significantly, as discussed in the atrophy section below.
Finally, although NAGM MTR mean and peak location decreased more than those in the NAWM over 1 year, NAWM MTR was a better baseline predictor of subsequent clinical change. This may indicate an imbalance in the changes in each segment prior to the start of the study: if NAWM changes occurred earlier in the disease course, they would initially contribute more to the clinical picture. Davies and colleagues39 found greater longitudinal decrease in NAGM compared with NAWM MTR in early relapsing and remitting MS patients, and backward extrapolation of their data suggested that NAWM changes had begun prior to symptom onset. If NAWM changes preceded NAGM changes in our cohort, we would expect the NAGM changes evident in this study to affect clinical outcome in subsequent years. Indeed, a recent MTR study on patients with other types of MS found that GM peak height changes predicted worsening of EDSS over 8 years,25 and examination of patients at a more advanced stage of PPMS (mean disease duration 10 years) using diffusion tensor imaging has shown that damage to the GM predicts disability at 5 years.40 Thus the relationship between changes in NAGM MTR and clinical change may be clarified by further follow‐up of this cohort.
MTR changes and progression of atrophy
Weak to moderate correlations emerged between MTR and volume changes over 1 year. Our data confirmed previous findings41,42: decrease in MTR peak height parameters showed the strongest association with the development of intrasegmental atrophy, with an increase in voxels with a lower MTR widening and flattening the normalised histogram.6 Statistically, however, atrophy explained less than 10% of the significant NAGM MTR changes which occurred in the mean and peak location of the histogram, and only 17% of significant NAWM changes (see table 4).
Our results emphasise that there is a modest relationship between atrophy and MTR, and that in patients with PPMS, MTR measures must be interpreted in the context of atrophy. However, we have demonstrated that MTR is an independent marker of pathology, as significant MTR changes over 1 year were not accounted for by brain volume changes, and baseline MTR measures predict clinical outcome independently of atrophy.
Acknowledgements
We would like to thank all participants in this study, and also Mara Cercignani, Daniel Tozer and Daniel Altmann for help and advice. We are grateful to the MS Society of Great Britain and Northern Ireland for funding this research. OC is a Wellcome Trust Advanced Fellow, and JSG is supported by the Spanish Ministry of Health (BEFI #02/9115).
Abbreviations
3D FSPGR - three dimensional inversion prepared fast spoiled gradient recall
BPF - brain parenchymal fraction
EDSS - Expanded Disability Status Scale
GM - grey matter
MS - multiple sclerosis
MSFC - Multiple Sclerosis Functional Composite
MT - magnetisation transfer
MTI - magnetisation transfer imaging
MTR - magnetisation transfer ratio
NABT - normal appearing brain tissue
NAGM - normal appearing grey matter
NAGMF - normal appearing grey matter fraction
NAWM - normal appearing white matter
NAWMF - normal appearing white matter fraction
NHPT - nine hole peg test
PASAT - paced auditory serial addition test
PH - peak height
PL - peak location
PPMS - primary progressive multiple sclerosis
pu - per cent units
TWT - timed walk test
WB - whole brain
WM - white matter
zNHPT - z score for the nine hole peg test
zPASAT - z score for the paced auditory serial addition test
zTWT - z score for the timed walk test
Footnotes
Competing interests: None.
References
- 1.Thompson A. Overview of primary progressive multiple sclerosis (PPMS): similarities and differences from other forms of MS, diagnostic criteria, pros and cons of progressive diagnosis. Mult Scler 200410(Suppl 1)S2–S7. [DOI] [PubMed] [Google Scholar]
- 2.Ingle G T, Stevenson V L, Miller D H.et al Primary progressive multiple sclerosis: a 5‐year clinical and MR study. Brain 20031262528–2536. [DOI] [PubMed] [Google Scholar]
- 3.Filippi M, Rovaris M, Rocca M A. Imaging primary progressive multiple sclerosis: the contribution of structural, metabolic, and functional MRI techniques. Mult Scler 200410(Suppl 1)S36–S44. [DOI] [PubMed] [Google Scholar]
- 4.van Waesberghe J H, Kamphorst W, De Groot C J.et al Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol 199946747–754. [DOI] [PubMed] [Google Scholar]
- 5.Schmierer K, Scaravilli F, Altmann D R.et al Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol 200456407–415. [DOI] [PubMed] [Google Scholar]
- 6.Tofts P.Quantitative MRI of the brain. Chichester: John Wiley & Sons, 2004
- 7.Ramio‐Torrenta L, Sastre‐Garriga J, Ingle G T.et al Abnormalities in normal appearing tissues in early primary progressive multiple sclerosis and their relation to disability: a tissue specific magnetisation transfer study. J Neurol Neurosurg Psychiatry 20067740–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson A J, Montalban X, Barkhof F.et al Diagnostic criteria for primary progressive multiple sclerosis: a position paper. Ann Neurol 200047831–835. [PubMed] [Google Scholar]
- 9.Kurtzke J F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983331444–1452. [DOI] [PubMed] [Google Scholar]
- 10.Cutter G R, Baier M L, Rudick R A.et al Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 1999122(Pt 5)871–882. [DOI] [PubMed] [Google Scholar]
- 11.Barker G J, Tofts P S, Gass A. An interleaved sequence for accurate and reproducible clinical measurement of magnetization transfer ratio. Magn Reson Imaging 199614403–411. [DOI] [PubMed] [Google Scholar]
- 12.Plummer D. Dispimage: a display and analysis tool for medical images. Rev Neuroradiol 19925489–495. [Google Scholar]
- 13.Chard D T, Parker G J, Griffin C M.et al The reproducibility and sensitivity of brain tissue volume measurements derived from an SPM‐based segmentation methodology. J Magn Reson Imaging 200215259–267. [DOI] [PubMed] [Google Scholar]
- 14.Perneger T V. What's wrong with Bonferroni adjustments. BMJ 19983161236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellison G W, Myers L W, Leake B D.et al Design strategies in multiple sclerosis clinical trials. The Cyclosporine Multiple Sclerosis Study Group. Ann Neurol 199436(Suppl)S108–S112. [DOI] [PubMed] [Google Scholar]
- 16.Hoogervorst E L, Eikelenboom M J, Uitdehaag B M.et al One year changes in disability in multiple sclerosis: neurological examination compared with patient self report. J Neurol Neurosurg Psychiatry 200374439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoogervorst E L, Kalkers N F, Uitdehaag B M.et al A study validating changes in the multiple sclerosis functional composite. Arch Neurol 200259113–116. [DOI] [PubMed] [Google Scholar]
- 18.Greenhalgh T. How to read a paper. Papers that report diagnostic or screening tests. BMJ 1997315540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Audoin B, Fernando K T, Swanton J K.et al Selective magnetization transfer ratio decrease in the visual cortex following optic neuritis. Brain 20061291031–1039. [DOI] [PubMed] [Google Scholar]
- 20.Chard D T, Griffin C M, Parker G J.et al Brain atrophy in clinically early relapsing‐remitting multiple sclerosis. Brain 2002125327–337. [DOI] [PubMed] [Google Scholar]
- 21.Tombaugh T N. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT). Arch Clin Neuropsychol 20062153–76. [DOI] [PubMed] [Google Scholar]
- 22.Solari A, Radice D, Manneschi L.et al The multiple sclerosis functional composite: different practice effects in the three test components. J Neurol Sci 200522871–74. [DOI] [PubMed] [Google Scholar]
- 23.Hobart J, Freeman J, Thompson A. Kurtzke scales revisited: the application of psychometric methods to clinical intuition. Brain 2000123(Pt 5)1027–1040. [DOI] [PubMed] [Google Scholar]
- 24.Hobart J, Kalkers N, Barkhof F.et al Outcome measures for multiple sclerosis clinical trials: relative measurement precision of the Expanded Disability Status Scale and Multiple Sclerosis Functional Composite. Mult Scler 20041041–46. [DOI] [PubMed] [Google Scholar]
- 25.Agosta F, Rovaris M, Pagani E.et al Magnetization transfer MRI metrics predict the accumulation of disability 8 years later in patients with multiple sclerosis. Brain 20061292620–2627. [DOI] [PubMed] [Google Scholar]
- 26.Filippi M, Bozzali M, Horsfield M A.et al A conventional and magnetization transfer MRI study of the cervical cord in patients with MS. Neurology 200054207–213. [DOI] [PubMed] [Google Scholar]
- 27.Rovaris M, Filippi M, Minicucci L.et al Cortical/subcortical disease burden and cognitive impairment in patients with multiple sclerosis. AJNR Am J Neuroradiol 200021402–408. [PMC free article] [PubMed] [Google Scholar]
- 28.Lucchinetti C, Bruck W. The pathology of primary progressive multiple sclerosis. Mult Scler 200410(Suppl 1)S23–S30. [DOI] [PubMed] [Google Scholar]
- 29.Davies G R, Altmann D R, Hadjiprocopis A.et al Increasing normal‐appearing grey and white matter magnetisation transfer ratio abnormality in early relapsing‐remitting multiple sclerosis. J Neurol 20052521037–1044. [DOI] [PubMed] [Google Scholar]
- 30.Fernando K T, Tozer D J, Miszkiel K A.et al Magnetization transfer histograms in clinically isolated syndromes suggestive of multiple sclerosis. Brain 20051282911–2925. [DOI] [PubMed] [Google Scholar]
- 31.Rovaris M, Gallo A, Valsasina P.et al Short‐term accrual of gray matter pathology in patients with progressive multiple sclerosis: an in vivo study using diffusion tensor MRI. Neuroimage 2005241139–1146. [DOI] [PubMed] [Google Scholar]
- 32.Kutzelnigg A, Lassmann H. Cortical lesions and brain atrophy in MS. J Neurol Sci 200523355–59. [DOI] [PubMed] [Google Scholar]
- 33.Kidd D, Barkhof F, McConnell R.et al Cortical lesions in multiple sclerosis. Brain 1999122(Pt 1)17–26. [DOI] [PubMed] [Google Scholar]
- 34.Bruck W, Stadelmann C. The spectrum of multiple sclerosis: new lessons from pathology. Curr Opin Neurol 200518221–224. [DOI] [PubMed] [Google Scholar]
- 35.Geurts J J. Imaging cortical lesions and NAGM at high and standard field strength: combined post‐mortem MRI and histopathology. ECTRIMS conference 2005, Thessaloniki, Greece, 28 Sept–1 Oct 2005, S8, abstract 40. (4.7T and 1.5T)
- 36.Geurts J J, Bo L, Pouwels P J.et al Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. AJNR Am J Neuroradiol 200526572–577. [PMC free article] [PubMed] [Google Scholar]
- 37.Kutzelnigg A, Lucchinetti C F, Stadelmann C.et al Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 20051282705–2712. [DOI] [PubMed] [Google Scholar]
- 38.Sastre‐Garriga J, Ingle G T, Chard D T.et al Grey and white matter volume changes in early primary progressive multiple sclerosis: a longitudinal study. Brain 20051281454–1460. [DOI] [PubMed] [Google Scholar]
- 39.Davies G R, Altmann D R, Hadjiprocopis A.et al Increasing normal‐appearing grey and white matter magnetisation transfer ratio abnormality in early relapsing‐remitting multiple sclerosis. J Neurol 20052521037–1044. [DOI] [PubMed] [Google Scholar]
- 40.Rovaris M, Judica E, Gallo A.et al Grey matter damage predicts the evolution of primary progressive multiple sclerosis at 5 years. Brain 20061292628–2634. [DOI] [PubMed] [Google Scholar]
- 41.Rovaris M, Bozzali M, Rodegher M.et al Brain MRI correlates of magnetization transfer imaging metrics in patients with multiple sclerosis. J Neurol Sci 199916658–63. [DOI] [PubMed] [Google Scholar]
- 42.Phillips M D, Grossman R I, Miki Y.et al Comparison of T2 lesion volume and magnetization transfer ratio histogram analysis and of atrophy and measures of lesion burden in patients with multiple sclerosis. AJNR Am J Neuroradiol 1998191055–1060. [PMC free article] [PubMed] [Google Scholar]