Abstract
We investigated olfactory function and its relation to cardiac 123I‐metaiodobenzylguanidine (MIBG) uptake in 15 patients with drug induced parkinsonism (DIP). The mean Cross Cultural Smell Identification (CCSI) score was significantly greater in patients with DIP than in those with Parkinson's disease (PD: 6.9 (1.6) vs 4.4 (2.2); p<0.001); however, the mean CCSI score in patients with DIP was not significantly different from controls. One patient with DIP, whose CCSI score was significantly reduced, also exhibited decreased cardiac MIBG uptake. DIP patients with CCSI scores within the normal range had normal cardiac MIBG uptake. Our study suggests that an olfactory function test may be a useful tool for detecting DIP unrelated to PD and for identifying patients with DIP who have subclinical PD.
The clinical manifestations of drug induced parkinsonism (DIP), which constitutes 15–60% of all parkinsonism cases, are very similar to those of Parkinson's disease (PD).1,2 Parkinsonian symptoms usually resolve within a few weeks after the patient stops taking the offending drugs. However, DIP is not always reversible; it has been reported that PD can develop after apparent recovery from DIP and that PD can persist and eventually worsen after discontinuation of the offending drug.3,4,5
It is well known that patients with PD have markedly impaired olfactory function.6 Furthermore, an impairment in olfactory function is an early manifestation of the neuropathological process in PD, and it may precede the development of parkinsonian motor symptoms.7 Recently, we have shown that the cardiac 123I‐metaiodobenzylguanidine (MIBG) uptake ratio is a useful tool for the differentiation of DIP from PD and the identification of DIP patients in whom the offending drugs may have unmasked clinical manifestations of parkinsonism.8
In this study, we investigated olfactory function in patients with DIP to assess its usefulness in differentiating PD from DIP, and evaluated its relation to cardiac MIBG uptake in patients with DIP.
Patients and methods
We prospectively enrolled 15 patients with DIP, 24 patients with PD and 15 healthy controls. All patients were referred to our hospital consecutively for the treatment or diagnostic evaluation of parkinsonism. DIP was diagnosed using the following three criteria: (1) presence of at least two of the four cardinal signs (tremor, rigidity, bradykinesia and impaired postural reflexes), (2) absence of a history of extrapyramidal disorders before treatment with the offending drug and (3) onset of symptoms during the course of treatment with the offending drug. All patients with DIP were examined every month for at least 3 months after withdrawal of the offending drug. None of the patients with DIP in the previous study8 were included in this study. PD was diagnosed according to the UK Parkinson's Disease Society Brain Bank clinical diagnosis criteria.9 The clinical stages of parkinsonism were assessed according to the classification of Hoehn and Yahr.10
Subjects suffering from otorhinolaryngeal disorders (upper respiratory tract infection or sinonasal disease, as established by otorhinolaryngologist),11 those exhibiting evidence of dementia (Mini‐Mental State Examination score <27)12 and current smokers13 were excluded from the study. None of the patients had a history of neuropathy, previous relevant cardiac disease or severe head injury. Routine chest radiography and electrocardiography revealed no abnormalities. Age matched subjects with no history of neurological or heart diseases were enrolled as a control group. All subjects provided written informed consent, and the study was approved by our hospital ethics committee.
To assess olfactory function, the Cross Cultural Smell Identification (CCSI) test,14 a widely used test of odour identification involving a scratch and sniff test of 12 microencapsulated odorants with a forced choice of four alternatives per item, was administrated to all subjects. For the cardiac uptake experiments, 123I‐MIBG (111 mBq) was injected intravenously into each subject, and cardiac uptake was imaged 3 h later, using a dual head γ camera system (MultiSPECT III, Siemens Medical Systems, Inc., Iselin, New Jersey, USA). The regions of interest were the whole heart and the mediastinum of the front image, and the ratio of 123I‐MIBG uptake in the heart to that in the mediastinum (H/M ratio) was calculated. The Mann–Whitney U and Fisher's exact tests were used to compare the means of groups in pairs when the variables were continuous and categorical, respectively. A p value of <0.05 was regarded as statistically significant. The statistical analyses were performed using commercially available software (SPSS, version 12.0).
Results
There was no significant difference in age between patients with DIP (68.7 (6.9)), patients with PD (64.4 (10.7)) and controls (63.7 (10.1)). The female to male ratio was not significantly different between patients with DIP (80%), patients with PD (50%) and controls (56%). There was no significant difference in the duration of education and the Mini‐Mental State Examination score between patients with DIP (12.5 (3.3) and 27.9 (1.1)), patients with PD (11.8 (4.1) and 27.8 (1.0)) and controls (13.8 (2.9) and 27.9 (0.9)). Mean duration of parkinsonism in patients with PD and DIP was 3.9 (SD 3.1) and 1.5 (SD 1.3) years, respectively. The mean follow‐up period in patients with DIP was 5.5 (SD 1.4) months. Details of the DIP patients and the offending drugs are summarised in table 1.
Table 1 Demographic features in patients with drug induced parkinsonism and their offending drugs.
| Patient No | Age (y)/ sex | Offending drug (duration, mo) | Initial H&Y | Follow‐up H&Y (mo)* | Patient No | Age (y)/ sex | Offending drug (duration, mo) | Initial H&Y | Follow‐up H&Y (mo)* |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 78/F | Levosulpiride (8) | 3 | 1 (5) | 9 | 59/F | Levosulpiride (14) | 2.5 | 1 (8) |
| 2 | 74/F | Haloperidone (12) | 2.5 | 0 (4) | 10 | 68/M | Levosulpiride (46) | 3 | 1 (6) |
| 3 | 65/F | Flunarizine (6) | 3 | 0 (6) | 11 | 66/F | Metoclopramide (8) | 2 | 0 (5) |
| 4 | 51/F | Perphenazine (30) | 2.5 | 0 (8) | 12 | 62/F | Risperidone (30) | 3 | 2 (6) |
| 5 | 73/M | Levosulpiride (38) | 3 | 1 (5) | 13 | 76/F | Levosulpiride (7) | 2 | 0 (5) |
| 6 | 63/F | Levosulpiride (46) | 2.5 | 0 (3) | 14 | 71/F | Levosulpiride (5) | 2 | 0 (6) |
| 7 | 67/M | Perphenazine (2) | 2.5 | 0 (4) | 15 | 73/F | Levosulpiride (7) | 2 | 2.5 (5) |
| 8 | 73/F | Levosulpiride (10) | 3 | 0 (7) |
H&Y, Hoehn and Yahr.
*The follow‐up period from the time the offending drug was discontinued to the time of the last follow‐up examination was performed in patients with drug induced parkinsonism.
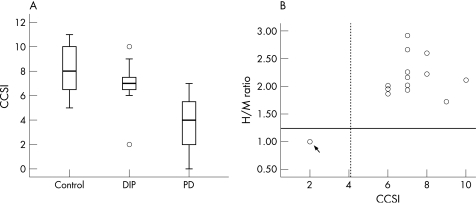
The mean CCSI score was significantly greater in patients with DIP than in those with PD (6.9 (1.6) (range 2–10) vs 4.1 (2.0) (range 0–7); p<0.001); however, the mean CCSI score in patients with DIP was not statistically different from that of controls (8.1 (1.9) (range 5–11)) (fig 1A). Fourteen of the 15 patients with DIP had a CCSI score within 2 SDs of the normal mean, but one score was more than 2 SDs below the normal mean and fell within the range of the PD patients. The relation between the CCSI score and H/M ratio is shown in fig 1B. One patient with DIP (No 15; arrow in fig 1B), whose CCSI score was more than 2 SDs below the normal range, also exhibited decreased cardiac MIBG uptake, which fell within the range of the PD patients (1.24 (0.11)). For the remaining DIP patients whose CCSI scores were within the normal range, the H/M ratios were within 2 SDs of the normal mean (2.06 (0.33)).
Figure 1 (A) Median and quartiles (box plots) as well as 10th and 90th percentiles (whiskers) of individual Cross Cultural Smell Identification (CCSI) scores in patients with drug induced parkinsonism (DIP), compared with patients with Parkinson's disease (PD) and controls. (B) The relation between the CCSI score and the heart to mediastinum ratio (H/M ratio) of 123I‐metaiodobenzylguanidine (123I‐MIBG) uptake in patients with DIP. Thick line in (A) indicates the mean value. Broken and continuous lines in (B) indicate the mean of CCSI score and H/M ratio in patients with PD, respectively.
The 14 patients with DIP whose CCSI scores were within the normal range exhibited considerable improvement or complete resolution of parkinsonism on clinical follow‐up after withdrawal of the offending drug, whereas the one DIP patient (No 15) with a significantly lower CCSI score exhibited persistent parkinsonism (table 1). This patient responded well to treatment with levodopa, showing a 63% improvement in the motor unified Parkinson's disease rating scale.
Discussion
The present study demonstrated that the mean CCSI score was significantly greater in patients with DIP than in those with PD, suggesting that an olfactory function test may be useful for differentiating patients with DIP from those with PD. Additionally, one DIP patient with a lower CCSI score also had decreased cardiac MIBG uptake, indicating that the offending drug may have unmasked clinical manifestations of parkinsonism in this patient
Depending on the clinical outcome, the patients with DIP can be classified as DIP unrelated to PD, DIP unmasking PD and DIP antedating PD.15 According to previous reports,4,5,8 most patients with DIP (70–90%) showed permanent resolution of parkinsonism after withdrawal of the offending drug, consistent with DIP unrelated to PD. Interestingly, these patients had normal 18F‐DOPA and cardiac MIBG uptakes, reflecting no nigrostriatal or cardiac sympathetic dysfunction, both of which are in PD. The present study demonstrated that most patients with DIP (93%) had CCSI scores within the normal range and above the range of PD patient scores, and showed marked improvement of the parkinsonian features after withdrawal of the offending drug, suggesting DIP unrelated to PD. Thus we propose that an olfactory function test may be a useful tool to detect patients with DIP who are unrelated to PD.
Although the number of subclinical PD cases in patients with DIP is small, it is important to detect them, from the viewpoint of disease prognosis and treatment strategies. It is difficult to detect a patient with DIP who has subclinical PD on the basis of the reversibility of the parkinsonian features. The 18F‐DOPA positron emission tomography (PET) and cardiac MIBG scans may be useful for detecting subclinical PD in patients with DIP. Burn and Brooks5 studied 13 patients with DIP using 18F‐DOPA PET and identified nigral dysfunction in three patients. Recently, we conducted cardiac MIBG scans in 20 patients with DIP and identified cardiac sympathetic dysfunction in two patients.8 All patients who had abnormal 18F‐DOPA and cardiac MIBG uptakes showed persistent parkinsonism after withdrawal of the offending drug.
In the present study, one patient with DIP whose CCSI score lay within the PD range exhibited persistent parkinsonism after the offending drug was withdrawn. Interestingly, this patient showed decreased cardiac MIBG uptake, which fell within the range of PD patients. According to Braak's neuropathological staging system for PD,16 the olfactory system may represent one of the induction sites of neuropathological processes in PD. In fact, not only olfactory impairment but also cardiac sympathetic dysfunction were reported in incidental Lewy body disease,17,18 a presymptomatic phase of PD, and these two systems may be closely coupled in patients with PD.19 Thus this DIP patient may be in subclinical PD, and the offending drug may aggravate the potential dopaminergic defect, resulting in the unmasking of clinical manifestations of parkinsonism.
The olfactory function test is simpler to perform, less invasive and less expensive than cardiac MIBG or PET scanning. Our study suggests that an olfactory function test may be a useful tool to screen for patients with DIP unrelated to PD and to identify patients with DIP who have subclinical PD. Further study using functional imaging to detect nigrostriatal function and long term follow‐up are warranted to determine whether DIP patients with low CCSI scores may also have nigral pathology.
Acknowledgements
This work was supported by the Korea Science and Engineering Foundation through the Chronic Inflammatory Disease Research Centre, Ajou University, grant R13‐2003‐019 (to I Jou).
Abbreviations
DIP - drug induced parkinsonism
CCSI - Cross Cultural Smell Identification
H/M ratio - heart/mediastinum ratio
MIBG - metaiodobenzylguanidine
PD - Parkinson's disease
PET - positron emission tomography
Footnotes
Competing interests: None
References
- 1.Benito‐Leon J, Bermejo‐Pareja F, Morales‐Gonzalez J M.et al Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology 200462734–741. [DOI] [PubMed] [Google Scholar]
- 2.de Lau L M, Giesbergen P C, de Rijk M C.et al Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology 2004631240–1244. [DOI] [PubMed] [Google Scholar]
- 3.Hardie R J, Lees A J. Neuroleptic‐induced Parkinson's syndrome: clinical features and results of treatment with levodopa. J Neurol Neurosurg Psychiatry 198851850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephen P J, Williamson J. Drug‐induced parkinsonism in the elderly. Lancet 198421082–1083. [DOI] [PubMed] [Google Scholar]
- 5.Burn D J, Brooks D J. Nigral dysfunction in drug‐induced parkinsonism: an 18F‐dopa PET study. Neurology 199343552–556. [DOI] [PubMed] [Google Scholar]
- 6.Katzenschlager R, Lees A J. Olfaction and Parkinson's syndromes: its role in differential diagnosis. Curr Opin Neurol 200417417–423. [DOI] [PubMed] [Google Scholar]
- 7.Ponsen M M, Stoffers D, Booij J.et al Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol 200456173–181. [DOI] [PubMed] [Google Scholar]
- 8.Lee P H, Kim J S, Shin D H.et al Cardiac 123I‐MIBG scintigraphy in patients with drug induced parkinsonism. J Neurol Neurosurg Psychiatry 200677372–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes A J, Daniel S E, Kilford L.et al Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 199255181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoehn M M, Yahr M D. Parkinsonism: onset, progression and mortality. Neurology 196717427–442. [DOI] [PubMed] [Google Scholar]
- 11.Doty R L, Mishra A. Olfaction and its alteration by nasal obstruction, rhinitis, and rhinosinusitis. Laryngoscope 2001111409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djordjevic J, Jones‐Gotman M, De Sousa K.et al Olfaction in patients with mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2007 (online first: 4 Jan 2007, doi:10. 1016/j. neurobiolaging. 2006. 11. 014) [DOI] [PubMed]
- 13.Frye R E, Schwartz B S, Doty R L. Dose‐related effects of cigarette smoking on olfactory function. JAMA 19902631233–1236. [PubMed] [Google Scholar]
- 14.Double K L, Rowe D B, Hayes M.et al Identifying the pattern of olfactory deficits in Parkinson disease using the brief smell identification test. Arch Neurol 200360545–549. [DOI] [PubMed] [Google Scholar]
- 15.Tolosa E, Coelho M, Gallardo M. DAT imaging in drug‐induced and psychogenic parkinsonism. Mov Disord. 2003;18(Suppl 7)S28–S33. [DOI] [PubMed]
- 16.Braak H, Del Tredici K, Rub U.et al Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 200324197–211. [DOI] [PubMed] [Google Scholar]
- 17.Iwanaga K, Wakabayashi K, Yoshimoto M.et al Lewy body‐type degeneration in cardiac plexus in Parkinson's and incidental Lewy body diseases. Neurology 1999521269–1271. [DOI] [PubMed] [Google Scholar]
- 18.Ross G W, Abbott R D, Petrovitch H.et al Association of olfactory dysfunction with incidental Lewy bodies. Mov Disord 2006212062–2067. [DOI] [PubMed] [Google Scholar]
- 19.Lee P H, Yeo S H, Kim H J.et al Correlation between cardiac 123I‐MIBG and odor identification in patients with Parkinson's disease and multiple system atrophy. Mov Disord 2006211975–1977. [DOI] [PubMed] [Google Scholar]