The internal vertebral venous plexus (IVVP) is part of the vertebral venous system (VVS). This system is the main alternative venous pathway, which joins, parallels but at the same time bypasses the caval venous system. The clinical relevance of the VVS is underestimated. We report on a patient who presented with cauda equina compression syndrome that was caused by engorgement of the IVVP. IVVP engorgement resulted from bilateral iliac vein thrombosis for which congenital agenesis of the inferior vena cava (IVC) was diagnosed as the predisposing factor.
Case report
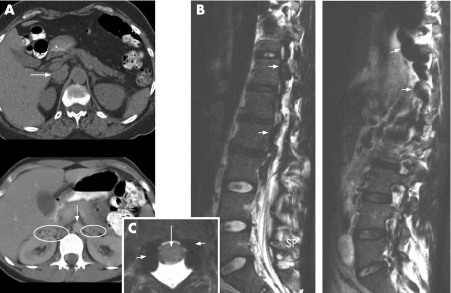
A 17‐year‐old female, with an unremarkable medical history, was referred to our hospital. She had suffered progressive lower back pain for 14 days and a cramp‐like burning sensation in her left leg for 11 days. In addition, she had paroxysmal paresthesias in both feet. On the day of referral she was unable to walk because of diffuse non‐radicular pain and weakness in both legs which developed within seconds after standing up. In this position, she also suffered from severe headache and vomiting. Physical examination revealed a body temperature of 37.6°C. Both inguinal fossae were tender to palpation. Neurological examination, including fundoscopy, was unremarkable. Laboratory tests revealed elevated C reactive protein (233 mg/ml (normal <4 mg/ml)), leucocytes (8.9×109/l (normal 4.0–10.0 ×109/l)) and thrombocytes (611 ×109/l (normal 150–350 ×109/l)). Echography confirmed the clinical suspicion of bilateral iliac vein thrombosis. CT of the abdomen (fig 1A) showed: (i) aplasia of the infrarenal IVC segment, (ii) bilateral thrombosis of the iliac veins and (iii) extensive dilatation/distension of the ovarian and renal veins as well as the external vertebral venous plexus (EVVP) and the azygos vein. MRI of the lumbar spine (fig 1B, C) showed cauda equina compression due to engorgement of the lumbar IVVP. Brain CT was normal.
Figure 1 (A) Upper picture shows a transversal CT scan of the abdomen of a normal subject. Arrow indicates the vena cava inferior. Lower picture shows transversal CT scan of the abdomen of the index case. Arrow indicates the descending aorta. Note the absence of the vena cava inferior. Ovals are drawn around dilated and thrombosed intra‐abdominal collateral renal and ovarian veins. (B) Sagittal T2 weighed MRI scan. Left picture shows a midsagittal view. Small arrows indicate dilated engorged internal vertebral venous plexus. Right picture shows a parasagittal view. Small arrows indicate dilated drainage vein into azygos. (C) Transversal T1 weighed MRI scan at the L1 level (patient is in the horizontal position, in this position there are no symptoms of cauda equina compression). Small arrows indicate enlarged veins, large arrow indicates the spinal cord.
It was concluded that the cauda equina compression was caused by engorgement of the lumbar IVVP in the presence of a congenitally compromised venous drainage of the lower limbs and small intestine due to IVC agenesis (IVCA). She was given warfarin which was replaced later by coumarine. Recovery was complete within 1 week. Oral contraceptives were discontinued. At follow‐up after 1 year, she had no complaints.
Discussion
IVCA was regarded as a major precondition for the lumbar IVVP engorgement that developed in our patient after iliac vein thrombosis. The latter caused an increased caudal inflow into the IVVP, while this venous pathway was already challenged by IVCA. In acute thrombosis of a normally developed IVC, congestion is the dominant cause of enlarged epidural veins. This may similarly provoke radicular pain. IVCA, which we demonstrated in our patient, is one of the developmental variations that may occur during the embryogenetic formation of the IVC in the sixth to eighth gestational weeks (incidence rates vary between 0.6 and 3.0%). IVCA is most frequently detected in the evaluation of deep venous thrombosis or as an incidental finding.
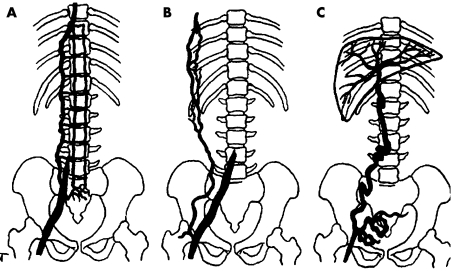
Alternative routes for venous return in the absence of IVC are, respectively, the (hemi‐) azygos system, the vertebrolumbar route, the anterior abdominal wall veins and the transumbilical portocaval route (fig 2). The vertebral route is involved in 95% of patients and is the sole route in 70% of such cases. Anterior abdominal wall veins are used in 15% of cases, almost always in addition to the vertebrolumbar route. The anterior abdominal wall venous pathway requires higher pressure to overcome venous valves and is only used if common iliac veins are thrombosed. Transumbillical portocaval shunting is found in 15% of patients and is the sole route in one‐third of those cases.1
Figure 2 Diagram showing the three main collateral pathways in obstruction or agenesis of the vena cava inferior. (A) Vertebrolumbar pathway. (B) Anterior abdominal wall pathway. (C) Transumbilical portocaval pathway. Adapted from Gorenstein and colleagues.1
In our patient, the infrahepatic segment of the IVC continued as the hemiazygos vein to the superior vena cava. Cauda equina compression and headache were logically explained by the functional anatomy of the VVS. The VVS is a valveless extradural plexiform venous network with a longitudinal pattern. It can be divided into three intercommunicating divisions: (1) the IVVP consists of veins surrounding the dura mater within the spinal canal (2), the basivertebral veins comprise the vessels within the vertebral bodies and (3) the EVVP surrounds the vertebral column.2 The IVVP extends the entire length of the vertebral column and finds a cranial terminus in the dural sinuses. The system communicates with the segmental (intercostal) veins of the thoracic–abdominal wall and with the azygos system and thus forms a “bypass” of the longitudinal veins of the abdomen and chest in the case of obstruction of the IVC (eg, thrombosis, tumour compression, pregnancy) or IVCA. The clinical relevance of the VVS as a vehicle for metastases of tumours originating in the small intestine along the spine has already been pointed out by Batson in 1940.3
The increase in symptoms in the erect position, which occurred in our patient, is compatible with the observations of Théron and Moret.4 They demonstrated that assuming an upright position is a prominent factor in directing blood flow through the VVS as an alternate route of cerebral venous return. In our patient, the IVVP was already engorged because of the IVCA and iliac vein thrombosis. In the upright position, venous return via the IVVP increases even more, thus inducing an increase in venous pressure in the cranial dural sinuses, which subsequently leads to increased intracranial venous pressure and headache.
Attention to pathology involving the VVS in the assessment of lumbar spine symptoms may improve diagnostic accuracy. For example, spinal epidural veins have been described that resembled prolapsed intervertebral discs on MRI, causing symptomatic nerve root compression. The correct diagnosis was only made during surgery. In this respect, Paksoy and Gormus5 have described a series of 13 out of 9640 patients who had radicular symptoms due to IVC obstruction or occlusion causing dilatation of VVS vessels and nerve root compression. De Kruijk et al presented a patient with acute cauda equina compression syndrome caused by dilated anterior epidural veins secondary to thrombosis of the IVC.6
In conclusion, we have emphasised the neurological relevance of knowledge of the VVS, and have aimed to achieve more awareness of the VVS for clinicians who are involved in diagnostic imaging of the lower spine. In particular, our case history indicates the importance of appreciating soft tissue signals on MRI of the spinal area.
Footnotes
Competing interests: None.
References
- 1.Gorenstein A I, Gordon R L, Shifrin E.et al Collateral pathways in inferior vena caval obstruction in children, including the porto‐caval route. Pediatr Radiol 198110225–228. [DOI] [PubMed] [Google Scholar]
- 2.Groen R J M, Groenewegen H J, van Alphen A M.et al Morphology of the human internal vertebral venous plexus: a cadaver study after intravenous Araldite CY 221 injection. Clin Neurol Neurosurg. 1997;99(Suppl 1)S178. [DOI] [PubMed]
- 3.Batson O V. The function of the vertebral veins and their role in the spread of metastases. 1940. Clin Orthop Relat Res. 1995;312:4–9/Arch Surg1940112138–149. [PubMed] [Google Scholar]
- 4.Théron J, Moret J.Spinal phlebography. Lumbar and cervical techniques. Berlin: Springer‐Verlag, 19783–23.
- 5.Paksoy Y, Gormus N. Epidural venous plexus enlargements presenting with radiculopathy and back pain in patients with inferior vena cava obstruction or occlusion. Spine 2004212419–2424. [DOI] [PubMed] [Google Scholar]
- 6.De Kruijk J, Korten A, Boiten J.et al Acute cauda equina syndrome caused by thrombosis of the inferior vena cava. J Neurol Neurosurg Psychiatry 199967827–828. [DOI] [PMC free article] [PubMed] [Google Scholar]