Abstract
Objective
X linked spinobulbar muscular atrophy (Kennedy disease (KD)), which is clinically characterised mainly by neuromuscular and endocrine symptoms, has to be considered as a multisystem disorder. Based on clinical evidence of central nervous system involvement, potential KD associated cerebral volume alterations were analysed in vivo.
Methods
Whole brain based analysis of optimised voxel based morphometry (VBM) was applied to three dimensional MRI data from 18 genetically confirmed KD patients and compared with age matched controls.
Results
Subtle decreases in grey matter volume, mainly localised in frontal areas, were found, but extensive white matter atrophy was observed, particularly in frontal areas, but also involving multiple additional subcortical areas, the cerebellar white matter and the dorsal brainstem from the midbrain to the medulla oblongata.
Conclusion
The VBM results demonstrated a morphological correlate of central nervous system involvement in KD, in agreement with aspects of the clinical phenotype (behavioural abnormalities, central–peripheral axonopathy) and with pathohistological findings.
Kennedy disease (KD), an X linked spinal and bulbar muscular atrophy, is caused by an expansion of a polymorphic tandem CAG repeat in the first exon of the androgen receptor (AR) gene.1 The clinical phenotype is characterised by an adult onset slowly progressive proximal and symmetrical weakness of the limb and bulbar muscles, muscular atrophy and generalised fasciculations, predominantly affecting the facial muscles.2 Additional symptoms mainly due to partial androgen insensitivity, postural tremor or frequent laryngospasms have been described. More subtle involvement of other neurological systems, such as the somatosensory or cognitive system, have been shown to be part of the clinical spectrum.1,3,4
To date, there have been no MRI studies investigating CNS structure in patients with KD. We used voxel based morphometry (VBM) to test the hypothesis that patients with KD have abnormal brain structural changes compared with controls. The VBM approach has been increasingly used as a powerful unbiased tool to investigate structural changes in three dimensional MRI of the whole brain in various neurodegenerative diseases.
Patients and methods
Patients
Ethics approval was given by the local ethics committee, and informed consent was obtained from all patients. We evaluated 18 men with genetically confirmed KD (mean age 49.9 (SD 8.4) years; mean number of CAG repeats 47.2 (2.4), range 42–50). Within the Caucasian only patient group, there were three groups of three brothers and one pair of first‐degree cousins. Mean age at manifestation of the disorder was 26.4 (11.5) years. Disease duration was 23.6 (10.1) years. All patients showed a typical clinical phenotype, including muscular spinobulbar tetraparesis, generalised fasciculations and gynaecomastia. Pareses, estimated by MRC grade, ranged between 3 and 5 in the upper limbs and between 3.5 and 4 in the lower limbs. Tremor of the hands was observed in 7/18 patients and pallhypaesthesia (<5/8 vibrations on the ankles) in 11/18. None of the patients showed obvious cognitive impairment compromising their social or professional activities. In previous neuropsychological screening, all patients had a normal IQ but showed deficits in frontal executive function and working and short time memory, in agreement with Guidetti et al.4 The control sample comprised 20 age matched healthy men (51.6 (11.2) years) with no history of abnormal medical conditions.
Data acquisition
High resolution three dimensional MRI data from all patients and controls were collected on the same 1.5 T clinical scanner (Siemens Symphony, Erlangen, Germany) under the same scanning protocol. T1 weighted magnetisation prepared rapid acquisition gradient echo data were acquired in the sagittal plane with the following parameters: 160–180 partitions, repetition time 9.7 ms, echo time 3.93 ms, flip angle 15°, matrix 256×256 mm2, field of view 250 mm and voxel size 1×1×1 mm3.
Data processing
Data processing followed the principles described by Good et al5 as the so‐called “optimised” VBM protocol, implemented in the Statistical Parametric Mapping software (SPM2, Wellcome Department of Imaging Neuroscience Group, London, UK; http://www.fil.ion.ucl.ac.uk/spm).
Within the framework of the general linear model, the patients' grey or white matter images were compared statistically with the control group's grey or white matter maps in a parametric group analysis for effects of diagnosis, including individual total grey or white matter volume and age as nuisance variables. Significance was set at p<0.05 corrected for multiple comparisons, using the false discovery rate algorithm.
Results
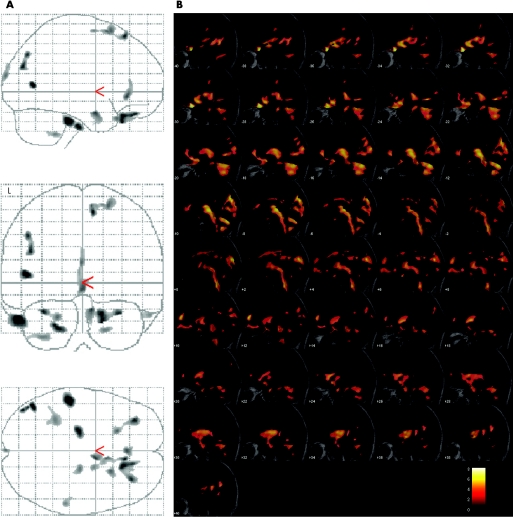
Eighteen men with a genetically confirmed diagnosis of KD were scanned using a three dimensional MRI protocol. The results for the grey and white matter analyses are given separately in table 1. In the analysis of regional grey matter volume, multiple atrophic areas were observed in both hemispheres (p<0.05 after correction for multiple comparisons). The volume changes were pronounced in frontal areas (bilateral orbital gyrus, right superior frontal gyrus) but were also observed in the inferior and middle temporal gyrus, limbic lobe, superior occipital gyrus and cerebellum (fig 1A). No increases in grey matter volume were found.
Table 1 Synopsis of the regional decreases of grey matter and white matter volumes.
| Anatomical localisation | MNI coordinates (mm) | p (FDR corrected) | T | No of voxels | |||
|---|---|---|---|---|---|---|---|
| Region | Hem | x | y | z | |||
| Grey matter | |||||||
| Inferior temporal gyrus | L | –54 | –19 | –30 | 0.012 | 6.47 | 1379 |
| Middle temporal gyrus | L | –47 | –67 | 8 | 0.012 | 6.15 | 437 |
| Orbital gyrus | L | –33 | 42 | –24 | 0.013 | 5.64 | 664 |
| Orbital gyrus | R | 16 | 28 | –25 | 0.013 | 5.63 | 1574 |
| Superior frontal gyrus | R | 9 | 30 | 58 | 0.014 | 5.41 | 730 |
| Superior occipital gyrus | L | –43 | –78 | 26 | 0.014 | 5.38 | 493 |
| Limbic lobe | R | 14 | –1 | –22 | 0.016 | 5.22 | 468 |
| Anterior cingulum | R | 1 | 33 | –5 | 0.017 | 5.07 | 643 |
| Superior frontal gyrus | R | 22 | 17 | 65 | 0.020 | 4.88 | 436 |
| Cerebellum | L | –34 | –43 | –45 | 0.025 | 4.11 | 596 |
| White matter | |||||||
| Frontal lobe (medial inferior) and brainstem | L | –30 | 26 | –16 | 0.000 | 8.23 | 102 432 |
| Cerebellum (medial) | L | –12 | –71 | –26 | 0.000 | 6.40 | 23 260 |
| Frontal lobe (medial) | R | 12 | 56 | 16 | 0.001 | 6.05 | 2253 |
| Temporal/occipital lobe | R | 40 | –55 | 17 | 0.002 | 4.74 | 1358 |
| Parietal lobe (near cingulum) | R | 2 | –55 | 27 | 0.003 | 4.40 | 4341 |
| Frontal lobe (mediofrontal) | R | 12 | 45 | –10 | 0.005 | 4.08 | 2656 |
| Frontal lobe (near precentral) | L | –29 | –22 | 50 | 0.009 | 3.77 | 691 |
| Temporal lobe (near fusiform) | R | 40 | –51 | –12 | 0.011 | 3.68 | 684 |
FDR, false discovery rate; Hem, hemisphere; MNI, Montreal Neurological Institute stereotaxic atlas.
Anatomical areas of grey matter and white matter regional atrophy, their localisation within the standardised stereotaxic atlas, their significance levels (expressed as p values and T scores) and the size of the atrophic areas (expressed as number of voxels) compared with healthy controls (thresholded at p<0.05, corrected for multiple comparisons).
Figure 1 (A) All areas with significant grey matter atrophy in the group analysis as an overlay on the standard glass brain template (sagittal, axial and coronal view; L, left). (B) Display of the areas showing significant white matter atrophy superimposed on the study specific template as a range of slices in the sagittal view, covering the brain from stereotaxic coordinates x = (−35) to x = +35, slice thickness 2 mm. Robustness is indicated by colour temperature according to the scale. After creation of a study specific template, the MRI data sets were normalised to this template and then segmented into grey matter, white matter and CSF. Voxel values in the segmented images were modulated by the Jacobian determinants of the transformation matrix, obtaining voxels that contained an absolute measure of tissue volume. The resulting grey and white matter maps were finally smoothed with a 6 mm isotropic Gaussian kernel.
In the white matter analysis, multiple large areas of highly significant regional volume loss were observed in a nearly symmetrical bi‐hemispheric pattern (table 1). Most prominently, subcortical areas in the bi‐hemispheric frontal lobe were included, in particular medial and medial inferior regions, including the areas with the highest robustness and very high numbers of voxels. In addition, atrophy was localised both in the subcortical areas of the temporal and the occipital lobe and in the parietal lobe (near the cingulum). Large white matter volume changes were also found in infratentorial regions (ie, in the cerebellar hemispheres and in the tectum of the midbrain). The dorsal brainstem also demonstrated marked decreases in white matter volume, including the medial lemniscus, spreading along the pons and the medulla oblongata until the caudal limits of the field of view (fig 1B). Regionally increased white matter volumes were not observed. The results (grey matter values or white matter values at the centres of significantly atrophic regions) did not correlate with CAG repeat number.
Discussion
In this voxel based three dimensional MRI analysis in 18 men with KD, marked regional brain atrophy was observed both in the frontal grey matter and in large white matter areas, including the frontal subcortical areas and the dorsal brainstem.
The present study is the first analysis of volume rendering MRI in KD, a disorder which is increasingly recognised as involving the CNS.4 Similar to amyotrophic lateral sclerosis, KD cannot be considered a pure motor neuron disorder. There is one previous report of frontal atrophy on CT scan in a patient with KD with clinical signs of frontal‐type dementia.6 Furthermore, frontobasal white matter abnormalities were found in histopathological case studies on KD.7 Volume of interest based MR spectroscopic studies of KD showed regional metabolic abnormalities only in motor cortex areas.8 The grey matter atrophy discovered in the current cohort of patients with KD is probably secondary to fibre tract alteration. The frontal distribution of atrophy correlates clinically with previous descriptions of frontal cognitive deficits in patients with KD.4,6,7
White matter changes were more prominent than expected and probably reflect damage to the subcortical fibre connections. These white matter changes have not been observed by VBM in other motor neuron diseases such as amyotrophic lateral sclerosis.9 White matter changes were more widespread than grey matter changes, but were more prominent in frontal regions. Areas adjacent to the motor cortex and in the cerebellum were also included, perhaps as a correlate of the slight (secondary) changes found in the motor cortex by MR spectroscopy.8
The dorsal brainstem was shown to be decreased in volume along its complete structure, demonstrating a pathological process which seems to continue into the cervical myelon. This finding was in line with the significant myelon atrophy at the cervical and thoracic level which was observed in patients with KD in a recent MRI study of the spinal cord.10 This finding was considered as a correlate of the KD specific central–peripheral distal axonopathy. It was demonstrated in the present study that continuation of this decrease in myelon size into the brainstem was associated with KD.
Our findings widen the phenotype associated with KD and indicate that extramotor areas of the CNS are also involved. Scans of individual patients would not have shown this; MRI analysis at the group level, such as VBM, is needed. The present results are not of diagnostic value per se as the clinical picture and molecular genetic test are apt to provide the diagnosis, but they add substantially to our understanding of the underlying pathomorphological processes in KD, together with the above mentioned upper spinal cord atrophy.10 The widespread subcortical involvement is in agreement with aspects of the clinical phenotype (cognitive deficits and behavioural abnormalities4). The patients should be referred for detailed neuropsychological testing, focusing on frontal and temporal function in addition to the previous clinical screening investigations. The findings are supported by the recent pathohistological findings of Adachi and colleagues11 (ie, extensive diffuse nuclear accumulation of mutant AR, including their presence in brainstem motor nuclei and spinal motor neurons, in addition to the well known neuron loss in the spinal cord and brainstem).12 This observation that diffuse nuclear mutant AR accumulation is of major pathogenetic importance in neuronal dysfunction and is closely linked to phenotypic expression13 has been known from other human polyglutamine diseases14 and from a transgenic mouse model of KD.15
Although correlation of the genotype and MRI changes (no significant correlation with CAG repeat number) and the relevance of the observed region specific alterations in the pathogenic process remain unresolved as yet, the widespread volume loss of the cerebral white matter is specific to KD. Neuroimaging techniques such as diffusion tensor imaging or MRI based correlation analyses with neuropsychological data may be promising in further investigations.
Abbreviations
AR - androgen receptor
KD - Kennedy disease
VBM - voxel based morphometry
Footnotes
Competing interests: None.
References
- 1.La Spada A R, Roling D B, Harding A E.et al Meiotic stability and genotype–phenotype correlation of the trinucleotide repeat in X‐linked spinal and bulbar muscular atrophy. Nat Genet 19922301–304. [DOI] [PubMed] [Google Scholar]
- 2.Sperfeld A D, Karitzky J, Brummer D.et al X‐linked bulbospinal neuronopathy: Kennedy disease. Arch Neurol 2002591921–1926. [DOI] [PubMed] [Google Scholar]
- 3.Buecking A, Pfister R. Sensory ataxia as the initial clinical symptom in X‐linked recessive bulbospinal neuronopathy. J Neurol Neurosurg Psychiatry 200069277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guidetti D, Vescovini E, Motti L.et al X‐linked bulbar and spinal muscular atrophy or Kennedy disease: clinical, neurophysiological, neuropathological, neuropsychological and molecular study of a large family. J Neurol Sci 1996135140–148. [DOI] [PubMed] [Google Scholar]
- 5.Good C D, Johnsrude I S, Ashburner J.et al A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 20011421–36. [DOI] [PubMed] [Google Scholar]
- 6.Kessler H, Prudlo J, Kraft S.et al Dementia of frontal lobe type in Kennedy's disease. Amyotroph Lateral Scler Other Motor Neuron Disord 20056250–253. [DOI] [PubMed] [Google Scholar]
- 7.Shaw P J, Thagesen H, Tomkins J.et al Kennedy's disease: unusual molecular pathologic and clinical features. Neurology 199851252–255. [DOI] [PubMed] [Google Scholar]
- 8.Mader I, Karitzky J, Klose U.et al Proton MRS in Kennedy disease: absolute metabolite and macromolecular concentrations. J Magn Reson Imaging 200216160–167. [DOI] [PubMed] [Google Scholar]
- 9.Kassubek J, Unrath A, Huppertz H J.et al Global brain atrophy and corticospinal tract alterations in ALS, as investigated by voxel‐based morphometry of 3‐D MRI. Amyotroph Lateral Scler Other Motor Neuron Disord 20056213–220. [DOI] [PubMed] [Google Scholar]
- 10.Sperfeld A D, Bretschneider V, Flaith L.et al MR‐pathologic comparison of the upper spinal cord in different motor neuron diseases. Eur Neurol 20055374–77. [DOI] [PubMed] [Google Scholar]
- 11.Adachi H, Katsuno M, Minamiyama M.et al Widespread nuclear and cytoplasmic accumulation of mutant androgen receptor in SBMA patients. Brain 2005128659–670. [DOI] [PubMed] [Google Scholar]
- 12.Sobue G, Hashizume Y, Mukai E.et al X‐linked recessive bulbospinal neuronopathy. A clinicopathological study. Brain 1989112209–232. [DOI] [PubMed] [Google Scholar]
- 13.Katsuno M, Adachi H, Doyu M.et al Leuprorelin rescues polyglutamine‐dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat Med 20039768–773. [DOI] [PubMed] [Google Scholar]
- 14.Schilling G, Wood J D, Duan K.et al Nuclear accumulation of truncated atrophin‐1 fragments in a transgenic mouse model of DRPLA. Neuron 199924275–286. [DOI] [PubMed] [Google Scholar]
- 15.Adachi H, Katsuno M, Minamiyama M.et al Heat shock protein 70 chaperone overexpression ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model by reducing nuclear‐localized mutant androgen receptor protein. J Neurosci 2003232203–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]