Abstract
Background and purpose
Posterior circulation stroke accounts for 20% of ischaemic strokes. Recent data suggest that the early stroke recurrence risk is high and comparable with carotid artery disease. Vertebral artery stenosis accounts for approximately 20% of posterior circulation stroke, and with endovascular treatment available accurate diagnostic imaging is important. We performed a systematic literature review to validate the accuracy of the non‐invasive imaging techniques Duplex ultrasound (DUS), magnetic resonance angiography (MRA) and computed tomographic angiography (CTA) in detecting severe vertebral artery stenosis, with intra‐arterial angiography (IAA) as the reference standard.
Methods
We identified studies that used non‐invasive imaging and IAA as the reference standard to determine vertebral artery stenosis and provided adequate data to calculate sensitivity and specificity. We analysed the quality of these studies, looked for evidence of heterogeneity and performed subgroup analysis for different degrees of stenosis.
Results
11 studies categorised stenosis into 50–99%. The sensitivity of CTA (single study) and pooled sensitivities of contrast enhanced MRA (CE‐MRA) and colour duplex were 100% (95% CI 15.8 to 100), 93.9% (79.8 to 99.3) and 70.2% (54.2 to 83.3), respectively. The specificities for CTA, CE‐MRA and colour duplex were 95.2% (83.8 to 99.4), 94.8% (91.1 to 97.3) and 97.7% (95.2 to 99.1). However, specificities for CE‐MRA and colour duplex demonstrated significant heterogeneity (p = 0.003 and p = 0.002, respectively).
Conclusions
CE‐MRA and possibly CTA may be more sensitive in diagnosing vertebral artery stenosis than DUS. However, data are limited and further high quality studies comparing DUS, MRA and CTA with IAA are required.
Posterior circulation stroke accounts for a fifth of strokes,1,2 and 20–25% of these are believed to be due to stenosis of a vertebral artery, with artery to artery embolism being the likely mechanism.3 Despite its apparent aetiological importance, optimal management of vertebral artery stenosis remains uncertain. This is in marked contrast with carotid stenosis for which the role of revascularisation with carotid endarterectomy has been established in large randomised controlled trials.4,5 Surgical revascularisation for vertebral artery stenosis is more complex because of the more difficult surgical access. Angioplasty and stenting are technically feasible, and no more difficult than carotid stenting, although their role in preventing recurrent posterior circulation stroke is uncertain.6 Progress in managing vertebral artery stenosis has been hampered by the traditional perception that vertebrobasilar strokes and transient ischaemic attacks (TIAs) have a benign prognosis compared with carotid territory ischaemic events. This has tended to make clinicians reluctant to investigate for vertebral stenosis, particularly when the role of revascularisation is uncertain. However, recent data demonstrate that the prognosis is far from benign and a systematic literature review has demonstrated that the risk of subsequent stroke is significantly higher in the acute phase of vertebrobasilar ischaemic events than carotid territory events.7
Non‐invasive imaging of vertebral stenosis is technically more complex compared with carotid stenosis. On Duplex ultrasound (DUS), most carotid stenoses can be clearly imaged, while only limited visualisation of the vertebral artery is possible. Until recently, the only alternative was intra‐arterial angiography (IAA) which remains the gold standard but carries a risk of iatrogenic stroke of approximately 1–2%.8 Non‐contrast magnetic resonance angiography (MRA) allows improved visualisation of the vertebral arteries, and more recently, contrast enhanced MRA (CE‐MRA) and contrast enhanced computed tomographic angiography (CTA) have been proposed as alternatives to the gold standard of IAA (fig 1). Many studies have compared these different imaging modalities for carotid artery stenosis. A recent meta‐analysis of carotid artery stenosis suggested CE‐MRA is more sensitive and specific than ultrasound, non‐contrast MRA and CTA.9 Fewer studies have compared these imaging modalities in vertebral artery stenosis. Furthermore, to extrapolate conclusions drawn for the carotid artery to the vertebral artery might be inappropriate, as the vertebral artery differs significantly anatomically from the internal carotid artery.
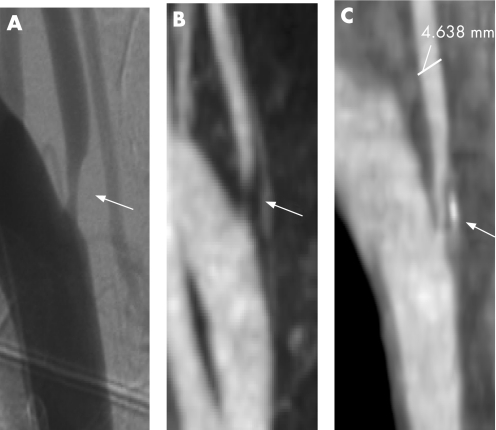
Figure 1 Intra‐arterial angiography (IAA), extracranial contrast enhanced magnetic resonance angiography (CE‐MRA) and computed tomographic angiography (CTA), demonstrating right vertebral artery stenosis in a 64‐year‐old patient who presented with a posterior circulation stroke. (A) IAA with right subclavian artery injection; (B) extracranial CE‐MRA maximum intensity projection image; (C) extracranial CTA sagittal reformatted image.
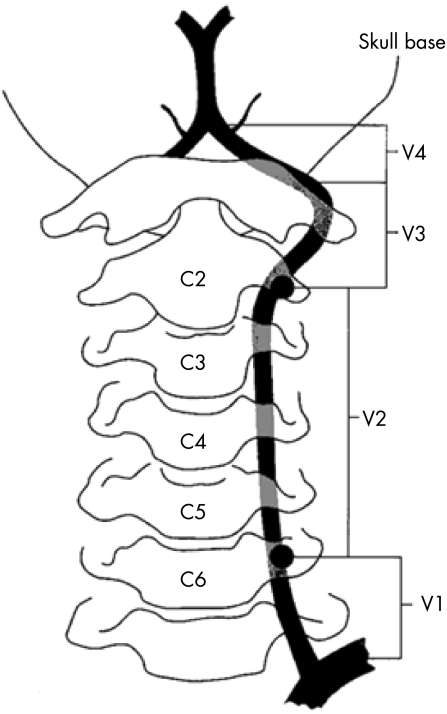
The vertebral artery is structurally divided into four sections (fig 2): V1–V3 form the extracranial vertebral artery and V4 forms the intracranial vertebral artery. The vertebral artery is much smaller (3–5 mm) than the internal carotid artery. It arises at right angles to its feeding vessel whereas the carotid artery arises directly from the common carotid artery. It is asymmetrical, with up to 15% of the population having one vertebral artery which is atretic. Approximately 50% have a dominant left vertebral, 25% a dominant right vertebral and 25% have both vertebral arteries of similar calibre.10
Figure 2 Schematic diagram illustrating the four segments of the vertebral artery
We conducted a systematic review of the literature to determine the diagnostic accuracy of DUS, both contrast and non‐contrast enhanced MRA, and CTA, in diagnosing vertebral artery stenosis or occlusion. Previous systematic analyses of carotid artery imaging have highlighted important methodological criteria by which such studies should be assessed.11,12 We used these criteria in assessing the quality of the vertebral artery imaging studies.
Methods
Data sources and study selection
We searched Medline, Embase and Pubmed (final search date 13 July 2006) for studies that used IAA as the reference standard to validate the accuracy of DUS, MRA and CTA to determine vertebral artery stenosis or occlusion.
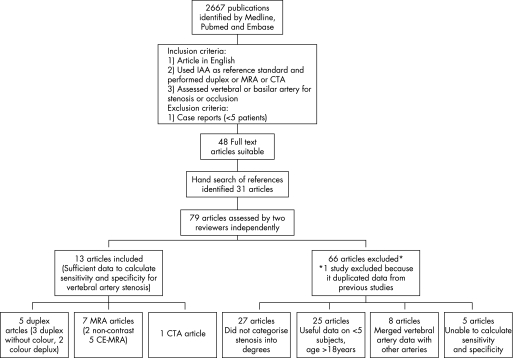
The search was limited to studies of humans and articles in English. We combined three search terms: (vertebral OR basilar OR posterior circulation OR vertebrobasilar) AND (magnetic resonance angiogram OR MRA OR magnetic resonance angiography OR computed tomographic angiogram OR computed tomographic angiography OR CTA OR duplex OR Doppler OR ultrasound OR ultrasonography OR angiogram OR angiography) AND (stenosis OR occlusion). Inclusion criteria were: (1) article in English; (2) used IAA as reference standard and performed DUS, MRA or CTA; and (3) assessed vertebral artery for stenosis or occlusion. Case reports (less than five patients) were excluded. Full text articles of abstracts fulfilling the criteria were reviewed. In addition, the references of articles which fulfilled the inclusion and exclusion criteria were hand searched (fig 3).
Figure 3 Flow diagram showing search methodology for study selection. CE‐MRA, contrast enhanced magnetic resonance angiography; CTA, computed tomographic angiography; DUS, IAA, intra‐arterial angiography; MRA, magnetic resonance angiography.
All articles which fulfilled the above criteria were independently assessed by two reviewers (GCC and SK) to identify those which provided sufficient data for inclusion in the analysis. Articles were excluded from the analysis if they: (1) did not categorise stenosis into degrees; (2) merged vertebral artery data with other vessels; (3) provided insufficient data to construct 2×2 contingency tables; or (4) provided these data on less than five patients over the age of 18 years. If data were only available for a subset of patients, this subset was included. If articles duplicated data, the article with the greatest amount of useful data was included.
Data extraction
The methodological quality of included articles was independently evaluated by the two reviewers (GCC and SK) on a standardised form. Criteria for data extraction were formulated from previous review articles11,12 and by discussion with a senior neuroradiologist (PR). The criteria included: demographic information (number of men/women, age (mean and range)), methodological quality (prospective, consecutive), patient disease group (posterior circulation stroke/TIA, anterior circulation stroke/TIA, healthy individuals, presumed dissection), number of patients in the study (number having non‐invasive imaging, IAA and number for which comparative data were provided), time interval between imaging, existence of verification bias, inclusion criteria for imaging, experience of radiologists reading the scans, blinded assessment, imaging technique (duplex, with or without colour, MRA (non‐contrast or CE‐MRA), CTA), scanning machine used (for MRI the Tesla number, for CT the slice scanner used), method of IAA angiography (selective, aortic arch, the planes imaged, eg, anteroposterior, lateral, oblique, etc) and method of derivation of stenosis. If it was not stated we assumed that the study was retrospective, non‐consecutive and not blinded. Disagreements were resolved by consensus or discussion with a third reviewer (HSM).
Analysis methodology
Sensitivity and specificity percentage values were calculated for several categories of stenosis: 50–99% versus <50% and 100%, 50–69/70% (depending on how this group was defined in studies) versus <50% and >70%, 70–99% versus <70% and 100% and 100% (occlusion) versus <100%. Summarising diagnostic accuracy is complex if the studies are heterogeneous; evidence of heterogeneity was sought by plotting diagnostic odds ratios (DOR) for all the stenosis groups and looking for evidence of outliers and performing χ2 heterogeneity testing. DOR is a measure of the discriminative power of diagnostic test results among diseased to the odds of a positive test result among non‐diseased. In order to calculate the DOR in studies which had 100% sensitivity or specificity values, 0.5 was added to all cells of the 2×2 diagnostic table.13
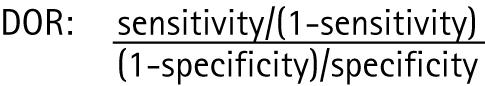 |
Subgroup analysis was performed for the different imaging modalities and degrees of stenosis by pooling data using a fixed effects model and searching for heterogeneity.
Results
A total of 3687 articles were identified using Medline, Pubmed and Embase (fig 3); 1023 were duplicate articles, leaving 2664 articles, and 2536 articles were excluded based on the inclusion and exclusion criteria. The full text of 128 articles was reviewed and of these, 48 fulfilled our criteria. A hand search of references identified a further 31 articles. These 79 articles were reviewed independently by the two reviewers. Twenty‐seven articles did not categorise data into degrees of stenosis, eight merged vertebral artery data with other vessels, five did not provide sufficient data to obtain sensitivity and specificity values and 25 articles did not provide comparative data on at least five patients over the age of 18 years. One article14 duplicated data from two previous studies.15,16 The author was contacted to check that the two studies did not overlap and the original articles, which had the greatest amount of data, were included.15,16 Two authors were contacted for further information on the number of arteries included in their studies; both had merged data in their articles making it impossible to calculate sensitivity and specificity values for vertebral arteries.17,18 Only one responded.17
The methodological quality of studies fulfilling the selection criteria was assessed and is presented in table 1.
Table 1 Important characteristics of the included studies.
| Author | Year subjects recruited | Disease group* | Imaging modality | No of subjects (arteries) | Prospective | Consecutive | Imaging technique stated | Blind assessment of imaging | Method of derivation of stenosis stated | Vessel compared | Degree of stenosis compared (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ackerstaff 198415 | 1981–1982 | E | Duplex without colour | 82 (103) | No | No | Yes | No‐Duplex | Yes | VAO | 1–99, 100 |
| Yes‐ IAA | |||||||||||
| Ackerstaff 198816 | 1983–1987 | E | Duplex without colour | Nm (239) | No | No | Yes | Yes | Yes | VAO, V1 | 0–49, 50–99, 100 |
| Visona 198625 | 1981–1983 | B, C, D | Duplex without colour | 25 (30) | Yes | No | No | No | Yes‐Duplex | VAO | 0, 50–99, 100 |
| No‐IAA | |||||||||||
| De Bray 200119 | 1996–1998 | A, B, D | Colour duplex | 158 (316) | Yes | Yes | Yes | Yes | Yes‐Duplex | ECVA | 0, 1–29, 30–49, 50–69, 70–99, 100 |
| No‐IAA | |||||||||||
| Harrer 200426 | NM | C, D | 2D and 3D Colour duplex | 6 (6) | No | No | Yes | Yes | Yes | VAO | <30, 30–69, >70 (raw data) |
| Wentz 199427 | NM | E | Non‐contrast MRA | 60 (161) | No | No | Yes | Yes | Yes | ICVA and BA data merged | 0, <50, 50–75, >75, 100 |
| Strotzer 199823 | NM | E | Non‐contrast MRA | 40 (80) | Yes | Yes | Yes | Yes‐MRA | No‐MRA | VAO | ⩾50 |
| No‐IAA | Yes‐IAA | ||||||||||
| Leclerc 199820 | 1997 | A, B | CE‐MRA 3D | 27 (50) | No | No | Yes | Yes | Yes | ECVA | 0, <50, 50–70, >70, 100 |
| Randoux 200324 | 2000–2001 | C | CE‐MRA | 33 (66) | Yes | Yes | Yes | Yes | Yes | VAO | 0,<50, >50, 100 |
| Cosottini 200321 | NM | A, B | CE‐MRA | 48 (89) | Yes | No | Yes‐MRA | Yes | No | ECVA | 0, <30, 30–70, >70, 100 |
| No‐IAA | |||||||||||
| Kim 200528 | NM | D, E | CE‐MRA | 37 (74) | Yes | No | Yes‐ MRA | No | Yes | ECVA | >50, 100 |
| No‐IAA | |||||||||||
| Yang 200517 | 2001–2002 | A, B, E | CE‐MRA | 40 (unclear) | No | No | Yes | Yes | Yes | ECVA, ICVA, BA | >50, 100 |
| Farres 199622 | NM | A | CTA | 24 (44 for V1, 33 for V0) | No | No | Yes | No | No | V0, V1 (incomplete data for V0) | <50, 50–70, >70, 100 |
BA, basilar artery; CE‐MRA, contrast enhanced magnetic resonance angiography; Consecutive, patients recruited consecutively into the study; CTA, computed tomographic angiography; ECVA, extracranial vertebral artery; IAA, intra‐arterial angiography; ICVA, intracranial vertebral artery; MRA, magnetic resonance angiography; NM, not mentioned; Prospective, prospective recruitment of patients; TIA, transient ischaemic attack; VAO, vertebral artery origin.
The number of vessels compared excludes the number of vessels not seen.
*Disease group: A = posterior circulation stroke or TIA, probably due to atheromatous disease; B = stroke or TIA, probably due to atheromatous disease (anterior circulation stroke or unspecified territory); C = risk factors for cerebrovascular disease; D = healthy individuals; E = other (including not mentioned, suspected atheromatous disease and neurological symptoms without stating vascular history).
The age of the study populations ranged from 18 to 77 years. Our inclusion criteria did not stipulate the aetiology of steno‐occlusive disease but we found that studies of dissection did not categorise stenosis into degrees. Five of the 13 studies recruited patients with posterior circulation stroke or TIA, probably secondary to atheromatous disease.17,19,20,21,22 Three studies were prospective and consecutive19,23,24 with two of these blindly assessing the imaging19,24 and one stating the method of derivation of stenosis for both the IAA and the non‐invasive imaging modality.24 Two studies did not provide details of the number of planes imaged during IAA to decide if accurate diagnosis of stenosis was possible.21,25 Eleven of the 13 studies provided comparison of 50–99% stenosis. This is analysed in detail and presented below.
Stenosis detection: 50–99%
Ultrasound
Three of the five ultrasound studies used duplex without colour to assess the vertebral artery origin.15,16,25 The duplex definition of stenosis differed in the three studies: Ackerstaff et al used antegrade direction of flow, with peak frequency >4 KHz, increased spectral broadening and striking turbulence during systole to define 1–99% stenosis.15 The same definition in Ackerstaff et al was used to define 50–99% stenosis16 and Visona et al defined 50–99% stenosis as high velocity signal >2 kHz with a broad spectrum, high pitched and harsh sound.25 Ackerstaff et al was not used for stenosis analysis but results were included for occlusion analysis.15 One study was prospective,25 one blindly assessed imaging techniques16 and all three recruited non‐consecutive patients. Pooled sensitivity, specificity and DOR were 70.2 % (95% CI 56.6 to 81.6), 93.4% (95% CI 89.2 to 96.3) and 37 (95% CI 16 to 83), respectively, for diagnosing 50–99% stenosis on duplex without colour versus diagnosing <50% stenosis or 100% (occlusion).
Two colour duplex studies were included in the analysis.19,26 The study of De Bray et al was a prospective, consecutive imaging study which blindly assessed the imaging results of 316 arteries,19 while the study of Harrer et al was a retrospective, non‐consecutive study blindly assessing the imaging of six arteries.26 Pooled sensitivity, specificity and DOR were 70.2% (95% CI 54.2 to 83.3), 97.7 (95% CI 95.2 to 99.1) and 75 (95% CI 24 to 234), respectively, for diagnosing 50–99% stenosis versus diagnosing <50% stenosis or 100% (occlusion).
MRA
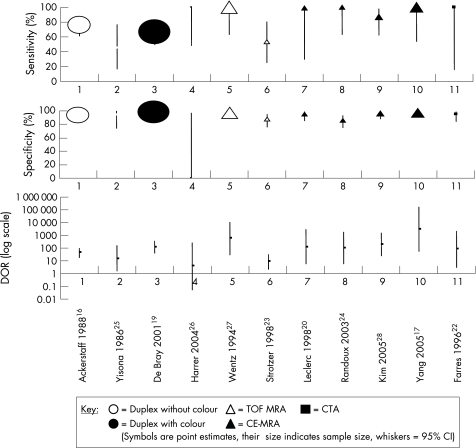
Two non‐contrast MRA studies were identified23,27 and these provided data for 50–99% stenosis which showed marked heterogeneity for sensitivity, specificity and DOR, demonstrating non‐overlapping DOR confidence intervals (fig 4) and significant χ2 heterogeneity (p = 0.007, p = 0.015 and p = 0.012, respectively). Wentz et al retrospectively examined 60 basilar and 106 intracranial vertebral arteries in an unspecified population and did not blindly determine the degree of stenosis.27 For 50–99% stenosis, it demonstrated sensitivity and specificity of 100% (95% CI 63.1 to 100) and 97.4% (95% CI 93.4 to 99.3), respectively. Strotzer et al prospectively recruited 40 consecutive patients and assessed the vertebral artery origin with two imaging techniques, coronal three dimensional fast imaging with steady state precession (3D FISP) and transverse 3D FISP.23 Data from the largest study group (3D FISP) were used in the analysis; this had not been blindly analysed. Data were provided for 63 vertebral arteries (17 arteries were not evaluable). It had poor sensitivity and specificity for 50–99% stenosis, 53.8% (95% CI 25.1 to 80.8) and 88% (95% CI 75.7 to 95.5), respectively.
Figure 4 Sensitivity, specificity and diagnostic odds ratio (DOR) comparing non‐invasive imaging techniques with intra‐arterial angiography in the diagnosis of 50–99% stenosis. CE‐MRA, contrast enhanced magnetic resonance angiography; CTA, computed tomographic angiography; IAA, intra‐arterial angiography; MRA, magnetic resonance angiography; TOF, time of flight.
Five CE‐MRA studies were identified.17,20,21,24,28 Three examined the extracranial vertebral artery,20,21,28 one the vertebral artery origin24 and one both the vertebral and basilar arteries.17 Four studies provided data for 50–99% stenosis,17,20,24,28 the largest CE‐MRA study categorised stenosis into 0, <30%, 30–70%, >70% and 100% and therefore could not be included in the 50–99% analysis but data from this study were used in the 70–99% and 100% (occlusion) analysis.21 The pooled sensitivity, specificity and DOR were 93.9% (95% CI 79.8 to 99.3), 94.8% (95% CI 91.1 to 97.3) and 179 (95% CI 42 to 765) with heterogeneity testing p values of 0.171, 0.002 and 0.127, respectively.
CTA
One CTA study fulfilling our criteria was identified.22 This study recruited 24 patients with a clinical diagnosis of vertebrobasilar ischaemia. It examined the vertebral artery origin (V0 and V1, separately), categorising stenosis into <50%, 50–70%, >70% and occlusion. For 50–99% stenosis it found sensitivity, specificity and DOR values of 100% (95% CI 15.8 to 100), 95.2% (95% CI 83.8 to 99.4) and 81% (3 to 2183.3), respectively.
Stenosis detection: 50–69/70% and 70–99%
Data on 50–69/70% and 70–99% stenosis were scarce (three and four studies, respectively) (table 2).
Table 2 Sensitivity, specificity and diagnostic odds ratio for 70–99% stenosis, 50–59/70% stenosis, 50–99% stenosis and occlusion in all imaging groups.
| Imaging | No of studies* | No of arteries | Sensitivity (95% CI) | Specificity (95% CI) | DOR (95% CI) |
|---|---|---|---|---|---|
| 70–99% stenosis | |||||
| Colour duplex | 1 | 316 | 65.2 (42.7 to 83.6) | 99.3 (97.5 to 99.9) | 272.8 (53.2 to 1398.1) |
| CE‐MRA | 2 | 139 | 83.3 (35.9 to 99.6) | 98.5 (94.7 to 99.8) | 200 (22 to 1824) |
| CTA | 1 | 44 | 100 (2.5 to 100) | 100 (91.8 to 100) | 261 (3.7 to 18197) |
| 50–69/70% stenosis | |||||
| Colour duplex (50–69%) | 1 | 316 | 61.5 (31.6 to 86.1) | 98.7 (96.7 to 99.6) | 119.6 (26.9 to 531) |
| CE‐MRA (50–70%) | 1 | 50 | 50 (1.3 to 98.7) | 95.8 (85.7 to 99.5) | 23 (1 to 517) |
| CTA (50–70%) | 1 | 44 | 100 (2.5 to 100) | 95.3 (84.2 to 99.4) | 49.8 (1.69 to 1562.1) |
| 50–99% stenosis | |||||
| Duplex without colour | 2 | 269 | 70.2 (56.6 to 81.6) | 93.4 (89.2 to 96.3) | 37 (16 to 83) |
| Colour Duplex | 2 | 322 | 70.2 (54.2 to 83.3) | 97.7 (95.2 to 99.1)† | 75 (24 to 34) |
| TOF MRA | 2 | 224 | 71.4(47.8 to 88.7)† | 95.1 (91.1 to 97.6)† | 22 (7 to 64)† |
| CE‐MRA | 4 | 263 | 93.9 (79.8 to 99.3) | 94.8 (91.1 to 97.3)† | 179 (42 to 765) |
| CTA | 1 | 44 | 100 (15.8 to 100) | 95.2(83.8 to 99.4) | 81 (3 to 2183.3) |
| 100% (occlusion) | |||||
| Duplex without colour | 3 | 372 | 98.8 (89.4 to 100) | 90.8 (87.2 to 93.7)† | 211 (38 to 1172) |
| Colour Duplex | 1 | 316 | 83.3 (51.6 to 97.9) | 100 (98.8 to 100) | 2557.8 (115.4 to 56671) |
| TOF MRA | 1 | 161 | 100 (75.3 to 100) | 100 (97.5 to 100) | 8019 (153 to 420402) |
| CE‐MRA | 4 | 278 | 89.5 (66.9 to 98.7) | 99.6 (97.9 to 100) | 429.7 (73.9 to 2498.6) |
CE‐MRA, contrast enhanced magnetic resonance angiography; CTA, computed tomographic angiography; DOR, diagnostic odds ratio; MRA, magnetic resonance angiography; TOF, time of flight.
*Where more than one study is available the results have been pooled using the fixed effects model
†Significant heterogeneity (χ2 testing, p<0.05).
For 50–69/70% stenosis, colour duplex and CE‐MRA had poor sensitivities of 61.5% (95% CI 31.6 to 86.1) and 50% (95% CI 1.3 to 98.7), respectively, with CTA having a high sensitivity but wide 95% confidence intervals (100% (95% CI 2.5 to 100)). The specificities for 50–69/70% were high for all three modalities: colour duplex 98.7% (95% CI 96.7 to 99.6), CE‐MRA 95.8% (95% CI 85.7 to 99.5) and CTA 95.3% (95% CI 84.2 to 99.4).
For 70–99% stenosis detection, sensitivities were slightly better than for 50–69/70%; colour duplex 65.2% (95% CI 42.7 to 83.6), CE‐MRA (pooled) 83.3% (95% CI 35.9 to 99.6) and CTA 100% (95% CI 2.5 to 100). The specificities were also better at 99.3% (95% CI 97.5 to 99.9), 98.5% (95% CI 94.7 to 99.8) and 100% (91.8 to 100), respectively.
Occlusion
Diagnosis of occluded arteries had the highest sensitivity, specificity and DOR. For occluded arteries, sensitivity of duplex without colour, colour duplex time of flight (TOF) MRA and CE‐MRA were 98.8% (95% CI 89.4 to 100), 83.3% (95% CI 51.6 to 97.9), 100% (95% CI 75.3 to 100) and 89.5% (95% CI 66.9 to 98.7). Specificities were 90.8% (95% CI 87.2 to 93.7), 100% (95% CI 98.8 to 100), 100% (95% CI 97.5 to 100) and 99.6% (95% CI 97.9 to 100). DOR were 211 (95% CI 38 to 1172), 2557.8 (95% CI 115.4 to 56671), 8019 (95% CI 153 to 420402) and 429.7 (95% CI 73.9 to 2498.6), respectively. The single CTA study did have one occluded artery at the origin but did not comment if this was seen on both CTA and IAA or only on a single imaging modality.22
Results from CTA studies not fulfilling our inclusion criteria
Several CTA studies were identified which used IAA to validate the accuracy of CTA but did not fulfil all of our inclusion criteria. These suggest that CTA may be as accurate29 or better that TOF MRA in detecting intracranial vertebral artery stenosis and occlusion.18 These were retrospective studies of 112 and 28 patients, respectively; both suggested that CTA might be superior to TOF MRA when slow flow is present. A prospective study in which patients were screened by MRA for >50% stenosis and then had CTA showed that the combination is equivalent to IAA.30
Discussion
Our systematic review demonstrated a scarcity of good quality studies validating the accuracy of diagnosing vertebral artery stenosis with non‐invasive imaging techniques against the gold standard of IAA. Some studies used a cut‐off of 70–99%, probably by analogy with carotid stenosis. The vertebral artery is however much smaller (3–5 mm) and this has led to many studies using 50–99% as their cut‐off point. Most data were available for 50–99% stenosis. Identification of the presence or absence of stenosis greater than 50% is important both in identifying vertebral stenosis as a cause of stroke and in identifying potential stenosis for further intervention. Here the available data suggested that CE‐MRA had the highest sensitivity followed by CTA, colour duplex and duplex without colour. The relevant DORs were 179.4 (95% CI 42 to 765), 81 (95% CI 3 to 2183), 75 (95% CI 24 to 234) and 37 (95% CI 16 to 83), respectively.
For carotid artery stenosis, the risk of stroke and the benefit of surgical intervention have been shown to depend on the degree of stenosis. Therefore, accurate assessment of the degree of stenosis is important, and 70% has been identified as the cut‐off above which patients particularly benefit from endarterectomy. Whether a similar cut‐off exists for vertebral artery stenosis, above which the risk of recurrent stroke is particularly high and there is potential benefit from intervention, remains to be determined. However, importantly, we identified few studies determining the accuracy of the different imaging modalities in identifying stenoses greater than 70%, and even fewer which determined the accuracy of quantifying the degree of stenosis in patients with stenosis. The limited data available suggested that for 70–99% stenosis, CTA and CE‐MRA are likely to be the optimal imaging techniques. Although colour duplex had a high DOR, this did not take into account false negatives which is important in a screening test. It is important to remember that as the vertebral artery is much smaller than the carotid artery, this is likely to reduce the accuracy of the stenosis estimation, particularly when determining stenosis to the nearest decile. In addition, when comparing non‐invasive techniques with IAA, it is important to be aware of the existence of significant interobserver and intraobserver variability when diagnosing the degree of stenosis. Kappa values of between 0.75 and 0.88 have been reported for interobserver agreement in the measurement of degree of carotid stenosis.31 We did not identify similar reproducibility studies for vertebral artery stenosis, but the smaller size of the vertebral artery may result in lower degrees of agreement.
The different imaging modalities offer different logistical advantages. Ultrasound is non‐invasive, cheaper and usually more readily available. Early studies used Doppler ultrasound alone but duplex ultrasound, which appears to have higher sensitivity in detecting vertebral stenosis, is now routine. Three of the five ultrasound studies which we included are over 20 years old and used duplex without colour which can be regarded as historical as it has been replaced with newer machines which use colour and have higher sensitivity and specificity. Our analysis suggests that DUS has a lower sensitivity than CE‐MRA and CTA. This is not surprising because ultrasound imaging cannot visualise the full length of the vertebral artery, and therefore detection of stenosis may have to rely on flow disturbance which is only present with more severe stenosis and does not directly show the site of stenosis. A further limitation of ultrasound is the difficulty of differentiating between dissection and atherosclerotic disease. MRA has the advantage that it can be combined with MRI, which has much greater sensitivity for detecting small posterior circulation infarcts. Initial non‐contrast MRA techniques offered less good visualisation of the vertebral artery than CE‐MRA, and did not always visualise the origin well, a common site of atherosclerosis. CE‐MRA has been shown to be more sensitive and specific for investigation of carotid artery stenosis and, from the limited data available, it appears to have higher sensitivity and specificity than either DUS or non‐contrast MRA for extracranial vertebral artery stenosis. It offers the advantage that skilled post processing is not necessary, but has a number of disadvantages including cost, contraindication in patients with metallic devices such as pacemakers and it is not tolerated because of claustrophobia in a minority of patients. In addition, particularly with administration of contrast, it is expensive. Multi‐slice CT scanning is more widely available and the limited data available suggested it offers a comparable sensitivity and specificity to CE‐MRA. It is cheaper and suitable for patients with contraindications to, or intolerance of, MRI. However, it is not without problems involving subjecting patients to radiation, and a potentially nephrotoxic contrast agent as well as being inaccurate for heavily calcified stenoses. Further studies are required to directly compare it with CE‐MRA.
The two non‐contrast MRA studies demonstrated significant heterogeneity in sensitivity (p = 0.007), specificity (p = 0.015) and DOR (p = 0.012) and were excluded from pooled analysis. The increased accuracy for TOF MRA may be explained by the fact that the TOF study imaged intracranial vessels and the 3D FISP study imaged vertebral artery origins. Measuring vertebral origins is much more challenging, the origins are often kinked and there is much more unavoidable movement because of pulsation from much larger vessels and breathing. Intracranial vessels are a different shape and apart from slight movement due to arterial pulsation, they are static during imaging. Although TOF MRA had the highest sensitivity, specificity and DOR for intracranial vessels, several studies have suggested that CTA is equivalent or better and recommend it over TOF MRA.18,29
The methodological quality of the studies was extremely varied. Whereas some were prospective consecutive studies with a significant number of arteries19 others were retrospective studies with only six arteries.26 The paucity of data did not allow comparison between the four different segments of the vertebral artery and it is likely that accuracy of the different imaging modalities varies according to the location of the stenosis. We included all of the studies and analysed the data. It is also known that not blindly assessing imaging overestimates the accuracy of imaging techniques.11 There were too few suitable studies to analyse the effect of other important factors such as type of scanning machine used, verification bias or publication bias. Another important factor which we were unable to address, because of lack of data, was the effect vertebral size has on the accuracy of stenosis detection in the different modalities. It is recognised that it can be difficult to differentiate between a severely stenotic and a hypoplastic vessel, although there is no consensus on the definition of a hypoplastic vessel with some studies using 2 mm and others 3 mm. None of the identified studies provided data for comparison of more than one non‐invasive imaging modality in the same patient population.
In conclusion, our systematic review demonstrates a paucity of high quality studies. From the data available, CE‐MRA appears to offer better sensitivity and specificity than duplex ultrasound for proximal vertebral artery stenosis. Despite CTA increasingly being used as the modality of choice to replace IAA in many centres, this technique still needs validation. Furthermore, no studies have compared the different imaging modalities against IAA in the same cohort of patients. Such studies are essential to determine optimal imaging protocols, particularly if patients with vertebral artery stenosis are to be selected for future randomised controlled trials of angioplasty and stenting.
Acknowledgements
We thank Dr Phil Rich for his assistance and Dr Ackerstaff for providing information. Dr Sofia Khan is supported by the Harrison fellowship from the Neurosciences Research Foundation.
Abbreviations
3D FISP - three dimensional fast imaging with steady state precession
CE‐MRA - contrast enhanced magnetic resonance angiography
CTA - computed tomographic angiography
DOR - diagnostic odds ratio
DUS - Duplex ultrasound
IAA - intra‐arterial angiography
MRA - magnetic resonance angiography
TIA - transient ischaemic attack
TOF - time of flight
Footnotes
Competing interests: None.
References
- 1.Bogousslavsky J, Van Melle G, Regli F. The Lausanne stroke registry: analysis of 1000 consecutive patients with first stroke. Stroke 1988191083–1092. [DOI] [PubMed] [Google Scholar]
- 2.Bamford J, Sandercock P, Dennis M.et al Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 19913371521–1526. [DOI] [PubMed] [Google Scholar]
- 3.Caplan L R, Amarenco P, Rosengart A.et al Embolism from vertebral artery origin occlusive disease. Neurology 1992421505–1512. [DOI] [PubMed] [Google Scholar]
- 4.European Carotid Surgery Trialists' Collaborative Group Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial. Lancet 19983511379–1387. [PubMed] [Google Scholar]
- 5.North American Symptomatic Carotid Endarterectomy Trialists' Collaborative Group The final results of the NASCET trial. N Engl J Med 19983391415–1425. [DOI] [PubMed] [Google Scholar]
- 6.Hankey G, Coward L, Featherstone R B R. Percutaneous transluminal angioplasty and stenting for vertebral artery stenosis. Stroke 2005362047–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flobman E, Rothwell P. Prognosis of vertebrobasilar transient ischaemic attack and minor stroke. Brain 20031261940–1954. [DOI] [PubMed] [Google Scholar]
- 8.Hass W K, Fields W S, North R R.et al Joint study of extracranial arterial occlusion. JAMA 1968203961–968. [PubMed] [Google Scholar]
- 9.Wardlaw J M, Chappell F M, Best J J K.et al Non‐invasive imaging compared with intra‐arterial angiography in the diagnosis of symptomatic carotid stenosis: a meta‐analysis. Lancet 20063671503–1512. [DOI] [PubMed] [Google Scholar]
- 10.Cloud G C, Markus H S. Diagnosis and management of vertebral artery stenosis. Q J Med 20039627–34. [DOI] [PubMed] [Google Scholar]
- 11.Rothwell P M, Pendlebury S T, Wardlaw J.et al Critical appraisal of the design and reporting of studies of imaging and measurement of carotid stenosis. Stroke 2000311444–1450. [DOI] [PubMed] [Google Scholar]
- 12.Nederkoorn P J, Van Der Graaf Y, Hunink M. Duplex ultrasound and magnetic resonance angiography compared with digital subtraction angiography in carotid artery stenosis. Stroke 2003341324–1332. [DOI] [PubMed] [Google Scholar]
- 13.Deville W L, Buntinx F, Bouter L M.et al Conducting systematic review of diagnostic studies: didactic guidelines. BMC Med Res Methodol 200229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jak J G, Hoeneveld H, Van der Windt J M.et al A six year evaluation of duplex scanning of the vertebral artery a non‐invasive technique compared with contrast angiography. J Vasc Technol 19891326–30. [Google Scholar]
- 15.Ackerstaff R G A, Hoeneveld H, Slowikowski J M.et al Ultrasonic duplex scanning in atherosclerotic disease of the innominate, subclavian and vertebral arteries. A comparative study with angiography. Ultrasound Med Biol 198410409–418. [DOI] [PubMed] [Google Scholar]
- 16.Ackerstaff R G A, Grosveld W J, Eikelboom B C.et al Ultrasonic duplex scanning of the prevertebral segment of the vertebral artery in patients with cerebral atherosclerosis. Eur J Vasc Endovasc Surg 19882387–393. [DOI] [PubMed] [Google Scholar]
- 17.Yang C W, Carr J C, Futterer S F.et al Contrast‐enhanced MR angiography of the carotid and vertebrobasilar circulations. Am J Neuroradiol 2005262095–2101. [PMC free article] [PubMed] [Google Scholar]
- 18.Bash S, Villablanca J, Jahan R.et al Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. Am J Neuroradiol 2005261012–1021. [PMC free article] [PubMed] [Google Scholar]
- 19.De Bray J M, Pasco A, Tranquart F.et al Accuracy of color‐Doppler in the quantification of proximal vertebral artery stenoses. Cerebrovasc Dis 200111335–340. [DOI] [PubMed] [Google Scholar]
- 20.Leclerc X, Martinat P, Godefroy O.et al Contrast‐enhanced three‐dimensional fast imaging with steady‐state precession (FISP) MR angiography of supraaortic vessels: preliminary results. Am J Neuroradiol 1998191405–1413. [PMC free article] [PubMed] [Google Scholar]
- 21.Cosottini M, Calabrese R, Puglioli M.et al Contrast‐enhanced three‐dimensional MR angiography of neck vessels: does dephasing effect alter diagnostic accuracy? Eur Radiol 200313571–581. [DOI] [PubMed] [Google Scholar]
- 22.Farres M T, Grabenwoger F, Magometschnig H.et al Spiral CT angiography: study of stenoses and calcification at the origin of the vertebral artery. Neuroradiology 199638738–743. [DOI] [PubMed] [Google Scholar]
- 23.Strotzer M, Fellner C, Fraunhofer S.et al Dedicated head‐neck coil in MR angiography of the supra‐aortic arteries from the aortic arch to the circle of Willis. Acta Radiol 199839249–256. [DOI] [PubMed] [Google Scholar]
- 24.Randoux B, Marro B, Koskas F.et al Proximal great vessels of aortic arch: comparison of three‐dimensional gadolinium‐enhanced MR angiography and digital subtraction angiography. Radiology 2003229697–702. [DOI] [PubMed] [Google Scholar]
- 25.Visona A, Lusiani L, Castellani V.et al The echo‐Doppler (Duplex) system for the detection of vertebral artery occlusive disease: comparison with angiography. J Ultrasound Med 19865247–250. [DOI] [PubMed] [Google Scholar]
- 26.Harrer J U, Wessels T, Poerwowidjojo S.et al Three‐dimensional color‐coded duplex sonography for assessment of the vertebral artery origin and vertebral artery stenoses. J Ultrasound Med 2004231049–1056. [DOI] [PubMed] [Google Scholar]
- 27.Wentz K U, Rother J, Schwartz A.et al Intracranial vertebrobasilar system: MR angiography. Radiology 1994190105–110. [DOI] [PubMed] [Google Scholar]
- 28.Kim S H, Lee J S, Kwon O K.et al Prevalence study of proximal vertebral artery stenosis using high‐resolution contrast‐enhanced magnetic resonance angiography. Acta Radiol 200546314–321. [DOI] [PubMed] [Google Scholar]
- 29.Skutta B, Furst G, Eilers J.et al Intracranial stenoocclusive disease: Double detector helical CT angiography versus digital subtraction angiography. Am J Neuroradiol 199920791–799. [PMC free article] [PubMed] [Google Scholar]
- 30.Hirai T, Korogi Y, Ono K.et al Prospective evaluation of stenoocclusive disease of the intracranial artery: combined MR angiography and CT angiography compared with digital subtraction angiography. Am J Neuroradiol 20022393–101. [PMC free article] [PubMed] [Google Scholar]
- 31.Young G, Humphrey P, Nixon T.et al Variability in measurement of extracranial internal carotid artery stenosis as displayed by both digital subtraction and magnetic resonance angiography. An assessment of three caliper techniques and visual impression of stenosis. Stroke 199627467–473. [DOI] [PubMed] [Google Scholar]