Abstract
Myelin protein zero (MPZ) is a major component of compact myelin in peripheral nerves where it plays an essential role in myelin formation and adhesion. MPZ gene mutations are usually responsible for demyelinating neuropathies, namely Charcot–Marie–Tooth (CMT) type 1B, Déjèrine–Sottas neuropathy and congenital hypomyelinating neuropathy. Less frequently, axonal CMT (CMT2) associated with MPZ mutations has been described. We report six patients (one sporadic case and five subjects from two apparently unrelated families) with a late onset, but rapidly progressive, axonal peripheral neuropathy. In all patients, molecular analysis demonstrated a novel heterozygous missense mutation (208C>T) in MPZ exon 2, causing the Pro70Ser substitution in the extracellular domain. The diagnosis of CMT2 associated with MPZ mutations should be considered in both sporadic and familial cases of late onset, progressive polyneuropathy. The mechanism whereby compact myelin protein mutations cause axonal neuropathy remains to be elucidated.
Myelin protein zero (MPZ, P0) is the major protein component of compact myelin in the peripheral nervous system. It plays a crucial role in myelin formation and adhesion, and is composed of three (extracellular, transmembrane and intracellular) domains.1 The gene coding for MPZ maps to chromosome 1p36 and has six exons. More than 100 mutations have been reported to date (see http://www.molgen.ua.ac.be/CMTMutations) which are usually associated with demyelinating neuropathies of different severity, namely Charcot–Marie–Tooth disease type 1B (CMT1B) and the more severe early onset Déjèrine–Sottas neuropathy and congenital hypomyelination.1 More rarely, however, MPZ mutations have been associated with the axonal CMT type 21,2,3 and the so‐called intermediate CMT.4
We describe six patients (one sporadic and five subjects from two apparently unrelated families) with a late onset, but rapidly progressive, axonal polyneuropathy caused by a novel mutation in the MPZ gene.
Patients and methods
The six patients belonged to three apparently unrelated families, originating from different parts of Lombardy (Northern Italy). In family A, the proband (A1), her younger brother (A2) and sister (A3), and their paternal aunt (A4) were affected, whereas her twin brother (A5) and father (who had died at age 52 years) were unaffected. The grandfather and a paternal aunt of family A proband, as well as 10 members from three generations of family B (including proband B1) were reported to have developed progressive walking difficulties after age 45–50 years. Family pedigree, with male to male transmission, is consistent with autosomal dominant inheritance for families A and B, whereas in family C only the proband (C1) was affected (his unaffected mother had died at age 42 years). The six affected patients underwent clinical and electrophysiological examination, with the exception of patient A4 who died before electrophysiology could be performed. Sural nerve biopsy was performed in two patients (B1 at age 67 years and C1 at age 55 years). Light and electron microscopy preparation as well as teased nerve fibre studies were performed according to standard methods. The density of myelinated fibres and their histograms were obtained on randomly chosen fields covering about 10% of the total transverse fascicular area using a semiautomatic program (Lucia measurement). A motor anconeus nerve biopsy was also performed in patient C1 at age 58 years.
Molecular study
Genomic DNA from six patients (table 1) and two unaffected relatives (A5 and B2, the daughter of patient B1) was analysed. Screening for mutations in the MPZ gene was performed by direct sequence analysis of the six exons and the flanking exon/intron boundaries on an automated DNA sequencer (Applied Biosystems, Foster City, California, USA).
Table 1 Clinical features and electrophysiological findings from nerve conduction studies.
| Patient No | Age (y)/ sex | Onset (y) | Muscle atrophy | Weakness | Sensory loss | CMTNS | |||
|---|---|---|---|---|---|---|---|---|---|
| Distal UL/LL | Prox UL/LL | Distal UL/LL | Prox UL/LL | PT | deep | ||||
| A1 | 71/F | 55 | +++ | –/+++ | ++/+++ | –/++ | ++ | +++ | 18 |
| A2 | 65/M | 52 | ++/+++ | – | ++/+++ | – | ↑ | – | 10 |
| A3 | 61/F | 45 | +/++ | –/++ | –/+++ | – | ++ | ++ | 15 |
| A4 | 79/F | 50 | +++/++ | –/++ | +/+++ | –/+ | ++ | ++ | na |
| B1 | 66/F | 47 | +/++ | – | –/+++ | –/+ | + | + | 12 |
| C1 | 62/M | 55 | +/+++ | –/+++ | +/+++ | – | ↑ | + | 13 |
| Age (y) | UL | LL | |||||||
| MCV (m/s) | CMAP (mV) | SCV (m/s) | SAP (μV) | MCV (m/s) | CMAP (mV) | SCV (m/s) | SAP (μV) | ||
| A1 | 60 | 51.7* | 16* | 50.0 | 7.50 | 34.3 | 0.3 | 44.2 | 3.2 |
| 67 | 51.6* | 12.6* | 50.0 | 2.2 | abs | abs | 38.7 | 2.0 | |
| 71 | 50* | 9.5* | 52.4 | 3.3 | abs | abs | abs | abs | |
| A2 | 54 | 47.9 | 6.5 | na | na | 38.1 | 0.3 | 41.7 | 6 |
| 65 | 49.0 | 8.9 | 46.9 | 4.7 | abs | abs | 40.0 | 5.7 | |
| A3 | 55 | 58.8 | 17.8 | na | na | abs | abs | 42.4 | 11 |
| 61 | 51.3 | 14.6 | 48.0 | 8.9 | abs | abs | 33.3 | 3.1 | |
| A4 | na | na | na | na | na | na | na | na | na |
| B1 | 66 | 53.7* | 13.3* | 60.3 | 3.4 | abs | abs | 39.6 | 2.2 |
| C1 | 62 | 51.6 | 13.4 | 49.4 | 4.0 | abs | abs | abs | abs |
Abs, no response recordable; CMAP, compound motor action potential; CMTNS, Charcot–Marie–Tooth Neuropathy Score5; Deep, position and vibration sense; LL, lower limbs; na, not assessed; MCV, motor conduction velocity; PT, pain‐touch; SAP, sensory action potential; SCV, sensory conduction velocity; UL, upper limbs.
Muscle atrophy, weakness, sensory loss: −, absent; +, mild; ++, moderate; +++, severe; ↑, hyperaesthesia.
For UL, values from the median nerve were recorded; *ulnar nerve; for LL, motor nerves, both peroneal and tibial nerves, were explored; values for patients A1 and A2 are from the peroneal nerve; LL sensory nerve values are from the sural nerves. Values in bold typeface are abnormal.
Results
Clinical data are summarised in table 1. All patients had a similar disease course with onset between the ages of 45 and 55 years. The disease started in the lower limbs with gait abnormalities and progressed rapidly. All patients developed wasting and weakness of the distal lower limb muscles, leading to complete foot drop within a few years. Involvement of the proximal lower limb muscles was observed in four patients. After a few years of the disease, all patients needed foot orthoses and four (A1, A4, B1, C1) needed a cane for walking. None had pes cavus. Four patients developed hand muscle weakness. Sensory involvement was milder. Pain was not a feature except for patient A3 who complained of foot pain. Deep tendon reflexes were absent in all patient except for A3 (decreased in upper limbs). No patient had pupillary abnormalities or other signs of autonomic nervous system involvement. Patient C1 underwent cardiovascular tests, which revealed normal functioning of the orthosympathetic and parasympathetic systems.
Five patients underwent electrophysiological studies (table 1) which showed a severe axonal neuropathy with marked abnormalities in the lower limb motor nerves (absent M response in almost all nerves) but normal compound motor action potentials in the upper limbs. Motor nerve conduction velocities were normal or only slightly reduced in the CMT2 range (47.9–58.8 m/s in the upper limb nerves). Sensory nerves were affected diffusely but to a lesser extent, with mildly to moderately reduced sensory action potential amplitudes in the upper and lower limbs and normal or slightly slowed sensory conduction velocities. Overall, the findings were consistent with a neuropathy affecting preferentially the lower limb motor fibres and causing severe axonal loss with only mild conduction impairment.
Molecular analysis
Direct testing for MPZ gene mutations was performed in the sporadic patient C1 and in the familial index cases A2 and B1. A novel heterozygous missense mutation (208C>T) was identified in MPZ exon 2, causing the substitution of proline‐70 with serine (Pro70Ser; Pro41Ser in the mature protein) in the extracellular domain. The mutation was subsequently found in all of the affected patients examined (table 1) but not in two relatives with normal clinical and electrophysiological examination (A5 and B2, daughter of patient B1, who asked for presymptomatic molecular diagnosis). The mutation was not found in 466 ethnically matched control subjects.
Nerve pathology
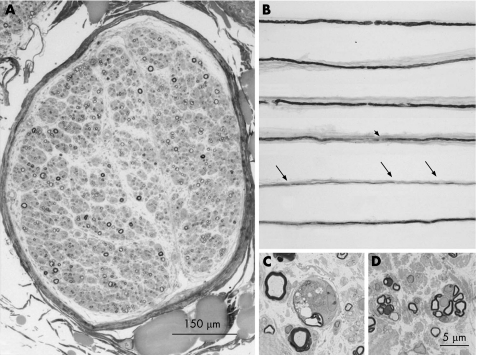
Sural nerve biopsy was performed in patients B1 (fig 1) and C1, and exhibited the characteristic pathological features of CMT2, showing a severe axonal neuropathy with occasional images of wallerian‐like degeneration and some clusters of regeneration. No evidence of demyelination, Schwann cell hypertrophy or hyperplasia (onion bulbs), myelin outfoldings, tomacula or other myelin abnormalities were detected at light or electron microscopy. There were fibres with thin myelin. On teased fibres, only signs of axonal degeneration and regeneration (fibres with homogeneous short internodes and remyelinated internodes clustered on single fibres) were evident (fig 1C). Morphometric examination disclosed severe reduction in fibre density (B1 = 3418/μm2; C1 = 4156/μm2; normal 7200–7600). The histogram of myelinated fibres was displaced towards the left, thus confirming a predominant loss of large myelinated fibres. Optic and electron microscopy examination of anconeus nerve biopsy (patient C1) revealed features similar to those of the sural nerve, with a moderate decrease in myelinated fibre density, clusters of regeneration, some fibres with thin myelin as compared to normal controls6 but no clear‐cut demyelination.
Figure 1 Sural nerve biopsy of patient B1 showed predominant axonal changes with loss of myelinated and unmyelinated fibres and regenerative clusters. Light microscopy. Transverse semithin sections (toluidine blue). (A) Nerve fascicle with severe reduction of myelinated fibre density. (C) Higher magnification: wallerian‐like degeneration. (D) Small myelinated fibres organised in “clusters”. (B) Teased fibres showing normal appearing internodes or irregular myelination at internodal sites (arrowhead), or short internodes (arrows).
Discussion
We have reported a novel mutation (Pro70Leu) in the MPZ gene causing an unusual form of CMT in three unrelated families. Constant and characteristic features were late onset of disease (age 45–55 years), rapidly progressive course (leading to the need for a walking aid within a few years) and electrophysiological and pathological evidence of chronic axonal involvement with only minor myelin abnormalities. The family history was consistent with autosomal dominant transmission in two families, but only one single case (C1) was present in the third family. For many years, because of the absence of family history and the presence of CSF oligoclonal bands (matched in the serum), this patient had been diagnosed as having an acquired neuropathy, unsuccessfully treated with steroids and immunosuppressants. The disease might be caused by a de novo mutation in this patient, although we cannot exclude the fact that his mother, who had died at age 42 years, would have developed CMT later in life.
In all patients examined, electrophysiological and nerve biopsy studies indicated a predominantly axonal neuropathy with only mild minor myelin changes. Motor nerve conduction velocities in all upper limb nerves were well above the limit of 38 m/s, which separates CMT1 from CMT2. In the early phases, lower limb nerves showed decreased compound motor action potentials, but either nerve conduction velocities were preserved or their decrease paralleled fibre loss. Later on, no M response was obtainable because of severe fibre loss. Sensory action potential amplitudes were consistently reduced with only minor sensory conduction velocity slowing. Nerve biopsy examination showed marked loss of axons, predominantly large fibres, regenerating clusters, with no significant myelin changes. Neither electron microscopy nor teased fibre examination disclosed clear‐cut signs of demyelination. However, thin myelin was a frequent finding indicating either regeneration or mild myelin involvement.
All of the available evidence indicates that this novel Pro70Ser mutation consistently leads to late onset and progressive CMT2. Other isolated mutations in the MPZ gene have been previously associated with the axonal type of CMT disease.1,2,7,8 The Thr124Met is the most common. It may cause the disease as late as 70 years of age and is associated with a wide range of nerve conduction velocities, mainly in the CMT2 range.3,9,10 Clinically, this form is characterised by a severe course, relevant sensory symptoms, including pain, frequent pupillary abnormalities, hearing loss and late dysphagia. Notably, late onset, pain and pupillary abnormalities are features commonly observed in CMT2 associated with MPZ mutations.1,2
Our patients, however, showed no sign of autonomic involvement or hearing loss, and sensory symptoms were overcome by motor ones. They all had a rapidly progressive neuropathy predominantly affecting the motor nerves of the lower limbs, with no pes cavus, and only the positive family history in two kinships was really suggestive of CMT. Because of the clinical similarities among these patients, we analysed MPZ in the sporadic case after having diagnosed the first family. It is possible that codon 70 is a mutational hotspot. However, as all three families were from a relatively homogeneous area (Lombardy) in Northern Italy, it seems likely that they share a common founder. Unfortunately, extended haplotype analysis could not be performed because of the limited number of subjects available for the study.
The Pro70Ser substitution is predicted by both the SIFT (http://blocks.fhcrc.org/sift/SIFT.html) and the PolyPhen (http://genetics.bwh.harvard.edu/pph/) algorithms to affect protein function. The mechanism whereby some mutations in the gene coding for the major compact myelin protein MPZ cause primary axonal degeneration rather than demyelination still remains to be understood. The Pro70Ser mutation is located in the extracellular domain, close to codon 75 (46 in the mature protein) where an Asp‐to‐Val substitution is also associated with CMT2.9 It is possible that mutations that do not dramatically change the characteristics of the protein are however able to interfere with its function, for example, altering Schwann cell–axon interactions and causing only subtle myelin abnormalities but relevant axonal damage. Interestingly, changes in inner myelin intralaminar and periaxonal spaces were found in the first autopsy performed in a CMT2 patient carrying a MPZ mutation (His39Pro–His10Pro in the mature protein, which causes a change in charge in the extracellular domain and is associated with late onset CMT).11
In conclusion, the identification of this novel mutation further confirms the crucial role of MPZ in the pathogenesis of axonal neuropathy. The available data strongly suggest that molecular analysis of the MPZ gene should be in the diagnostic investigations of patients with late onset, progressive axonal neuropathy, either familiar or sporadic.
Acknowledgements
The authors thank the families for their participation in this study.
Abbreviations
CMT - Charcot–Marie–Tooth
MPZ - myelin protein zero
Footnotes
Funding: This work was supported by Telethon and Telethon‐UILDM (grants GUP02169 to DP, GUP04009 to FT and GTF02008 to MM), Fondazione Pierfranco e Luisa Mariani (grant R‐05‐44 to FT), Associazione Amici del Centro Dino Ferrari‐University of Milan and Eurobiobank project QLTR‐2001‐02769 (grants to MM).
Competing interests: None.
References
- 1.Shy M E, Jani A, Krajewski K.et al Phenotypic clustering in MPZ mutations. Brain 2004127371–384. [DOI] [PubMed] [Google Scholar]
- 2.Shy M E. Peripheral neuropathies caused by mutations in the myelin protein zero. J Neurol Sci 200624255–66. [DOI] [PubMed] [Google Scholar]
- 3.De Jonghe P, Timmerman V, Ceuterick C.et al The Thr124Met mutation in the peripheral myelin protein zero (MPZ) gene is associated with a clinically distinct Charcot–Marie–Tooth phenotype. Brain 1999122281–290. [DOI] [PubMed] [Google Scholar]
- 4.Mastaglia F L, Nowak K J, Stell R.et al Novel mutation in the myelin protein zero gene in a family with intermediate hereditary motor and sensory neuropathy. J Neurol Neurosurg Psychiatry 199967174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shy M E, Blake J, Krajewski K.et al Reliability and validity of the CMT neuropathy score as a measure of disability. Neurology 2005641209–1214. [DOI] [PubMed] [Google Scholar]
- 6.Gates P, Byrne E, McKelvie P.et al Motor nerve biopsy: feasibility and safety. Clin Exp Neurol 19933033–38. [PubMed] [Google Scholar]
- 7.Bienfait H M, Faber C G, Baas F.et al Late onset axonal Charcot–Marie–Tooth phenotype caused by a novel myelin protein zero mutation. J Neurol Neurosurg Psychiatry 200677534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finsterer J, Miltenberger G, Rauschka H.et al Novel C59T leader peptide mutation in the MPZ gene associated with late‐onset, axonal, sensorimotor polyneuropathy. Eur J Neurol 2006131149–1152. [DOI] [PubMed] [Google Scholar]
- 9.Misu K, Yoshihara T, Shikama Y.et al An axonal form of Charcot–Marie–Tooth disease showing distinctive features in association with mutations in the peripheral myelin protein zero gene (Thr124Met or Asp75Val). J Neurol Neurosurg Psychiatry 200069806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurihara S, Adachi Y, Imai C.et al Charcot–Marie–Tooth families in Japan with MPZ Thr124Met mutation. J Neurol Neurosurg Psychiatry 2004751492–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Bai Y, Ianakova E.et al Major myelin protein gene (P0) mutation causes a novel form of axonal degeneration. J Comp Neurol 2006498252–265. [DOI] [PubMed] [Google Scholar]