Abstract
Background
While patients with amyotrophic lateral sclerosis (ALS) may complain of fatigue, the underlying mechanisms appear complex, with dysfunction of central and peripheral nervous systems independently reported as contributing factors. The aim of the present study was to further delineate the mechanisms underlying increased fatigability in ALS by measuring activity dependent changes in axonal excitability following a maximum voluntary contraction (MVC).
Methods
Nerve excitability changes were recorded before and after an MVC of the abductor pollicis brevis in 16 patients with ALS and 25 controls.
Results
In patients with ALS, there was a greater increase in threshold (36.5 (5.9)%; controls 19.6 (3.5)%; p<0.05) as a result of MVC, with reduction in the amplitude of the compound muscle action potential generated by a submaximal stimulus (ALS 49 (7.6)%; controls 41.0 (5.4)%). These changes were associated with an increase in superexcitability (ALS 65.1 (25.4)%; controls 42.3 (5.7)%) and reduction in strength–duration time constant (ALS 20 (4.9)%; controls 10 (2.5)%; p<0.01), indicative of axonal hyperpolarisation. The increase in threshold was more pronounced in patients with ALS with predominantly lower motor neuronal involvement.
Conclusions
Higher firing rates of surviving motor axons attempting to compensate for neurogenic weakness are likely to explain the greater activity dependent changes in ALS. As such, the present study suggests a further peripheral factor underlying the development of fatigue in ALS.
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder that affects motor neurones in the spinal cord, brainstem and motor cortex.1,2 The consequences of this neurodegeneration are motor deficits in the limbs, bulbar and respiratory muscles.3 Although the mechanisms of neuronal dysfunction, and ultimately the development of symptoms in ALS, remain unknown, glutamate excitotoxicity,4,5,6 increased levels of inducible nitric oxide synthase levels4 and, in cases of inherited ALS, oxidative stress secondary to mutations in the superoxide dismutase‐1 gene, have been proposed.7,8,9,10
Increased fatigability, defined as an inability to sustain a predictable maximal force during voluntary contraction, is a common symptom of ALS.11,12,13 The mechanisms underlying fatigue in ALS are complex, and contributions from both the central and peripheral nervous systems have been reported.11,12 Central fatigue refers to a reduced excitatory drive to motor neurones, secondary to central nervous system dysfunction, resulting in incomplete motor unit recruitment and submaximal motor unit discharge rates. In contrast, peripheral fatigue typically refers to impaired muscle activation, caused by dysfunction at or below the anterior horn cell.13,14 Perhaps somewhat counterintuitively, fatigue in ALS appears to be independent of muscle strength and disease severity.15,16 Regardless of the underlying mechanism, fatigue in ALS severely impacts on the patient's quality of life.15,16
The ability to sustain a motor output may be assessed by measuring changes in axonal membrane threshold following a voluntary contraction. Specifically, in peripheral nerves, voluntary contraction activates the axonal membrane Na+/K+ pump,17 which attempts to return the resting membrane potential to baseline after contraction has ceased,18,19,20,21 resulting in activity dependent hyperpolarisation. The magnitude of activity dependent hyperpolarisation is determined by the impulse load22 and, in neurological diseases where the safety margin for impulse conduction has been reduced as occurs for instance in demyelinating neuropathy, may be sufficient to induce conduction failure.23,24,25 In an attempt to further delineate the mechanisms underlying fatigability and weakness in ALS, the present study measured activity dependent changes in axonal excitability induced by voluntary contraction.
Materials and methods
Studies were undertaken in 16 patients with clinically probable or definite ALS, as defined by the revised El Escorial criteria.26 There was no history of other significant comorbidities, which may have caused neuropathy in these patients. Specifically, focal compressive mononeuropathies, such as median nerve dysfunction at the level of the wrist, were excluded in all patients. All patients were receiving riluzole at the time of the study, for which fatigue may be a potential side effect.27,28,29 Patients were referred from the multidisciplinary motor neurone disease clinic at the Prince of Wales Hospital and gave informed consent to the procedures, which were approved by the South East Sydney Area Health Service Human Research Ethics Committee (Eastern Section) and the Committee on Experimental Procedures Involving Human Subjects of the University of New South Wales.
Fatigue was assessed using the multidimensional fatigue inventory (MFI).30 The MFI is a 20 item self‐report instrument designed to measure five dimensions of fatigue, including general, physical and mental fatigue, reduced activity and reduced motivation. There are four items in each dimension, with the score for each item ranging from 1 (no fatigue) to 5 (severe fatigue), and the score for each dimension ranging from 4 (no fatigue) to 20 (fatigue). The total MFI score may range from 5 (no fatigue) to 100 (severe fatigue).
All patients with ALS were clinically staged using the ALS functional rating scale‐revised (ALSFRS‐R),31 Medical Research Council32 clinical grading of power in conjunction with Trigg's hand function score33 and forced vital capacity. Patients with ALS were classified according to site of disease onset as either limb or bulbar onset, and clinical phenotype as predominantly lower or upper motor neurone. Amplitude of the compound muscle action potential (CMAP) was used to differentiate patients with ALS with more severe disease, as reflected by mild–moderately reduced CMAP amplitude of <4 mV.34,35
In all studies, the median nerve was stimulated at the wrist and the resultant CMAP was recorded from the abductor pollicis brevis (APB) using surface electrodes. The active recording electrode was placed over the motor point of the APB and the reference was placed 4 cm distally, at the base of the thumb. The procedure lasted for 14 min, consisting of 3 min of baseline recording, 1 min of maximal voluntary contraction (MVC) and 10 min of recovery. Skin temperature was maintained at >32°C during voluntary contraction. Prior to excitability studies, CMAP amplitude and onset latency, F wave latency and frequency were measured. The neurophysiological index (NI) was derived according to a previously reported formula:
NI = CMAP amplitude (mV) × F wave frequency/distal motor latency (ms)
where F wave frequency was expressed as the number of F responses recorded with 20 stimulations of the median nerve at the wrist.36,37
Median nerve excitability was tracked before and after MVC of the APB for 60 s using a computerised threshold tracking program (Institute of Neurology, London, UK).37 Recordings were amplified (gain 1000, bandwidth 3 Hz to 3 kHz) and digitised using an analogue‐to‐digital board (DT2812, Data Translation, Marlboro, Massachusetts, USA), with a sampling rate of 10 kHz. Stimulus waveforms were converted to current with a purpose built isolated linear bipolar constant current stimulator.
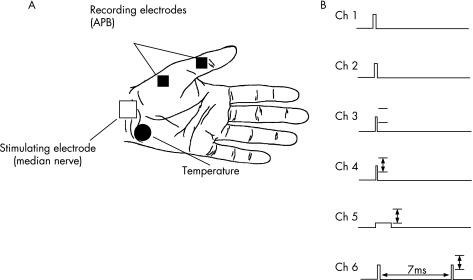
Stimuli were delivered at 0.8 s intervals and rotated sequentially through a series of six different conditioning test combinations (fig 1). On channel 1, a fixed supramaximal stimulus of 0.2 ms was delivered to produce a CMAP of maximal amplitude. A stimulus, 20% greater than channel 1, was delivered on channel 2 and commenced following the period of voluntary contraction. This was done in order to ensure that the post‐contraction maximal CMAP on channel 1 had remained truly maximal.23 On channel 3, a stimulus of 0.1 ms duration was delivered and its intensity fixed for the entire duration of the study to 70% of the precontraction maximal CMAP, in order to assess the effects of contraction induced excitability changes on CMAP amplitude.
Figure 1 Configuration of the testing paradigm. Vertical arrows indicate threshold tracking of test potential (set to 70% of the maximal potential, as measured on stimulus channel (Ch) 1). A supramaximal stimulus, 20% greater than stimulus channel 1, was delivered on stimulus channel 2. Stimulus channel 3 was of fixed intensity to produce a potential 70% of maximum. Unconditioned test stimuli of 0.1 ms and 1 ms duration were delivered on stimulus channels 4 and 5, respectively. On stimulus channel 6, an interstimulus interval of 7 ms was used to measure superexcitability. Stimuli were delivered at 1.2 Hz, rotating through the six stimulus channels sequentially. APB, abductor pollicis brevis.
On channels 4–6, proportional tracking was used to produce a target CMAP of 70% of maximal.38 On channels 4 and 5, 0.1 ms and 1 ms stimuli were used, respectively, to achieve the target response and from the data obtained the strength duration time constant (τSD) was calculated using Weiss' formula.39,40 Channel 6 tracked the changes in superexcitability, a period of increased axonal excitability which follows the relative refractory period and is due to passive discharge of the myelin sheath.41,42 To assess superexcitability, a conditioning supramaximal stimulus was delivered 7 ms before the test stimulus. The test response on this channel was measured after online subtraction of the conditioning stimulus obtained in isolation (using the response obtained from channel 1). Superexcitability was measured as the reduction in stimulating current required to produce the target CMAP and expressed as a negative threshold change.
During the period of voluntary contraction, subjects abducted the thumb against resistance and were encouraged to maintain maximal effort for the entire duration of the 60 s. Resistance was provided by the same person in all studies. Stimuli were not delivered during the period of contraction and the hand and forearm were stabilised to limit contraction of other muscle groups and to ensure that there was no displacement of electrodes.
Values were compared with data obtained from normal controls (n = 25, 14 males, mean age 45 (3.1) years (range 26–73)). Although the controls were younger than patients with ALS, subgroup analysis in healthy controls suggested that age did not significantly affect the activity dependent changes in threshold, as indicated by the fact that the threshold increase in younger controls (age <45 years, mean age 33.0 years, n = 14) of 1.20 (0.02)% was similar to that for the older group (age >45 years, mean age 60.3 years, n = 11) of 1.22 (0.07)% (p = 0.3). For comparison of the magnitude of activity dependent changes in excitability, patients with ALS were assigned by two blinded neurologists as having either predominantly lower motor neurone or upper motor neurone involvement, as measured by the upper motor neurone (UMN) score.43 Specifically, in patients with ALS with predominantly lower motor neurone involvement, the UMN score was ⩽7, while an UMN score of 8–16 indicated predominantly UMN involvement. Single comparisons in excitability parameters were analysed using the Student's t test. Repeated measures analysis of variance (ANOVA) was used for multiple comparisons, and correlations were analysed using Pearson's correlation coefficient. A p value of <0.05 was considered statistically significant. Results are expressed as mean (SEM).
Results
The clinical features of 16 patients with ALS are summarised in table 1. All patients reported fatigue, with the total MFI score ranging from 27 to 93. CMAP amplitude (ALS 6.5 (1.9) mV; controls 9.5 (0.5) mV, 95% CI controls 4.6 to 14.4 mV; p<0.05) and neurophysiological index (ALS 0.6 (0.1); controls 2.6 (0.2) mV, 95% CI controls 0.6 to 4.6; p<0.00001) were significantly reduced in patients with ALS. As previously reported,44 baseline τSD, which reflects activity of persistent Na+ conductance,45 was significantly longer in patients with ALS (ALS 0.45 ms (0.04); controls 0.36 (0.02), 95% CI controls 0.16 to 0.57; p<0.05). Baseline superexcitability was not significantly different between the two groups (ALS −18.3 (2.0)%; controls −17.3 (1.4)%; p = 0.3).
Table 1 Summary of clinical features in the 16 patients with amyotrophic lateral sclerosis from the present study.
| Patient No | Age (y)/ sex | ALS onset | Disease duration (months) | ALSFRS‐R | Trigg's hand score | MFI | MRC | FVC (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 50, M | UL | 24 | 42 | 0 | 40 | 5 | 100 |
| 2 | 53, F | UL | 12 | 39 | 2 | 93 | 4 | 146 |
| 3 | 55, M | UL | 41 | 34 | 2 | 58 | 4 | 98 |
| 4 | 69, M | UL | 6 | 45 | 1 | 81 | 4 | 52 |
| 5 | 60, F | UL | 8 | 46 | 1 | NQ | 4 | 100 |
| 6 | 64, M | UL | 26 | 36 | 2 | 81 | 4 | 57 |
| 7 | 69, M | UL | 17 | 40 | 2 | 44 | 5 | 67 |
| 8 | 67, F | UL | 16 | 40 | 2 | NQ | 4 | 85 |
| 9 | 44, F | UL | 13 | 46 | 2 | 50 | 4 | 79 |
| 10 | 71, M | UL | 99 | 42 | 1 | 81 | 4 | 90 |
| 11 | 46, M | LL | 9 | 42 | 1 | 48 | 5 | 62 |
| 12 | 69, M | LL | 29 | 36 | 0 | NQ | 4 | 89 |
| 13 | 65, M | Bulbar | 14 | 42 | 1 | 27 | 4 | 81 |
| 14 | 53, F | Bulbar | 50 | 44 | 0 | 29 | 5 | 57 |
| 15 | 66, M | Bulbar | 10 | 38 | 2 | 64 | 4 | 79 |
| 16 | 68, F | Bulbar | 21 | 36 | 0 | NQ | 5 | 80 |
| Mean | 60.6 | 24.7 | 40.5 | 1.2 | 58 | 4.3 | 82.6 | |
| SEM | 2.3 | 5.8 | 0.9 | 0.2 | 6.4 | 0.1 | 5.7 |
ALS, amyotrophic lateral sclerosis; ALSFRS‐R, amyotrophic lateral sclerosis functional rating scale‐revised; FVC, forced vital capacity; LL, lower limb; MFI, multidimensional fatigue inventory; MRC, Medical Research Council; NQ, not quantitated; UL, upper limb
The site of disease onset was classified as either UL, LL or bulbar.
Disease duration refers to the period from symptom onset to date of testing.
The patients were clinically graded using the ALSFRS‐R, with a maximum score of 48 when there is no disability. The ALSFRS‐R is comprised of four subscores; bulbar (maximum score 12), fine motor (maximum score 8), gross motor (maximum score 16) and respiratory (maximum score 12).
Hand function was assessed using the Trigg's hand function score.
In four patients the MFI was not quantitated at the time of excitability testing.
Muscle strength was clinically assessed using the MRC for abductor pollicis brevis, as this muscle was utilised for excitability testing.
FVC was also assessed in each patient as a marker of disease severity.
Activity dependent changes in axonal excitability
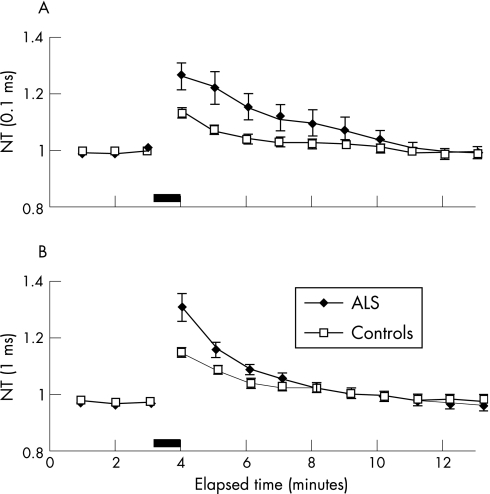
Following MVC, there was an increase in peak threshold in patients with ALS for stimuli of 0.1 and 1 ms duration, as illustrated in a representative ALS patient (fig 2A, B). Mean data revealed a significantly greater increase in threshold for 0.1 ms (peak threshold increase ALS 1.26 (0.04); controls 1.13 (0.01), 95% CI controls 1.03 to 1.23; p<0.05) and 1 ms (peak threshold increase ALS 1.38 (0.05); controls 1.19 (0.03), 95% CI controls 0.90 to 1.4; p<0.05) stimuli durations in patients with ALS compared with controls (fig 2A, B). Repeated measures ANOVA confirmed that the threshold remained significantly higher (p<0.05) in patients with ALS for the first 8 min with stimuli of 0.1 ms, and for the first 3 min for stimuli of 1 ms duration in the immediate post‐contraction period (fig 3A, B).
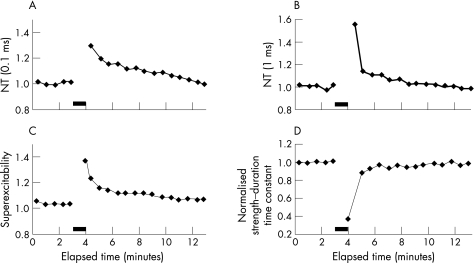
Figure 2 Traces demonstrating the increase in normalised threshold (NT) induced by a maximal voluntary contraction (MVC) for 60 s in a representative patient with amyotrophic lateral sclerosis for stimuli of (A) 0.1 ms and (B) 1 ms duration. The increase in threshold was accompanied by (C) an increase in superexcitability and (D) a reduction in the strength–duration time constant. These changes were consistent with the development of membrane hyperpolarisation. The filled bar represents the period of MVC.
Figure 3 Group data for 16 patients with amyotrophic lateral sclerosis (ALS) and 25 normal controls revealed a significantly greater increase in normalised threshold (NT) in patients with ALS for stimuli of (A) 0.1 ms and (B) 1 ms duration, following a maximum voluntary contraction (MVC) of 60 s duration. Threshold changes are expressed as mean (SEM). The filled bar represents the period of MVC.
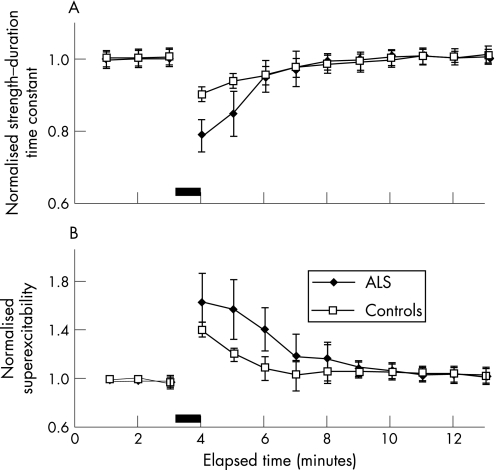
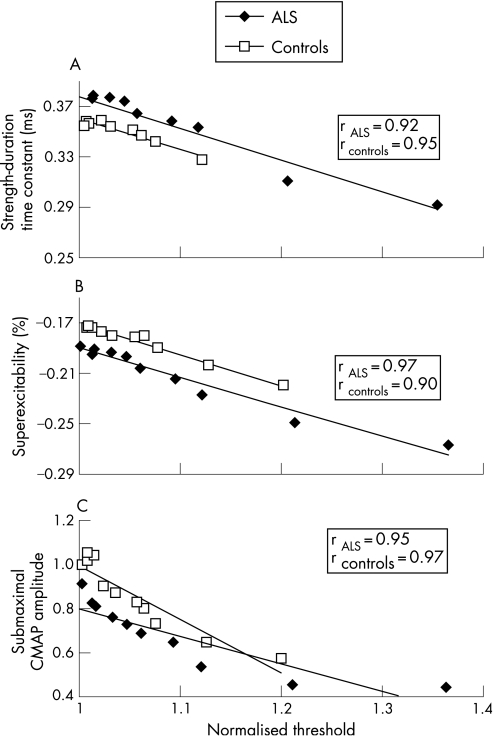
This increase in threshold was accompanied by a reduction in τSD (fig 2D) and an increase in superexcitability (fig 2C), as illustrated for a representative ALS patient. Group data confirmed a significant reduction in τSD in patients with ALS post‐MVC compared with controls (peak reduction in ALS 0.79 (0.04); controls 0.90 (0.02), 95% CI controls 0.70 to 1.10; p<0.01) (fig 4A). The reduction in τSD was accompanied by an increase in superexcitability in both ALS and normal controls following MVC (fig 4B). These changes in τSD and superexcitability were consistent with hyperpolarisation of the axonal membrane17,19 and correlated with an increase in threshold for both patients with ALS and controls (fig 5A, B).
Figure 4 Group data in 16 patients with amyotrophic lateral sclerosis (ALS) and 25 normal controls (A) revealed a significant reduction in the strength–duration time constant in patients with ALS compared with controls after a maximum voluntary contraction (MVC) of 60 s duration. (B) Superexcitability, a sensitive parameter of membrane potential, was increased in patients with ALS and controls after MVC. The filled bar represents the period of MVC.
Figure 5 The increase in threshold for stimuli of 1 ms duration correlated with (A) reduction in the strength–duration time constant, (B) an increase in superexcitability and (C) a reduction in the submaximal compound muscle action potential (CMAP) amplitude post maximum voluntary contraction, in patients with amyotrophic lateral sclerosis (ALS) and controls. The changes in these excitability parameters are consistent with membrane hyperpolarisation in patients with ALS and controls.
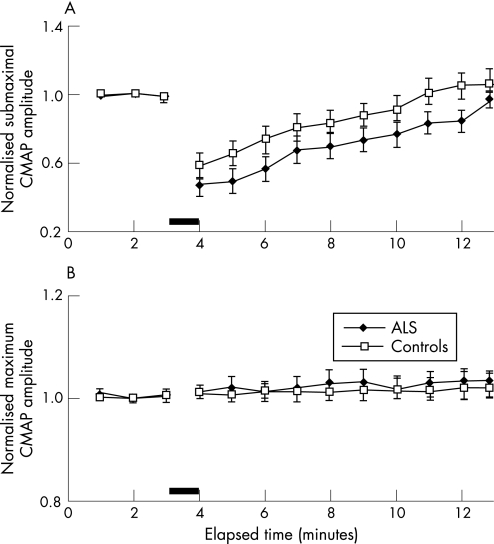
These changes in axonal excitability were accompanied by a reduction in the CMAP amplitude produced by a submaximal stimulus of fixed intensity (fig 6A). The reduction in submaximal CMAP amplitude correlated with the increase in threshold in patients with ALS and controls (fig 5C). However, there was no reduction in CMAP amplitude to a maximal stimulus in patients with ALS post MVC (fig 6B).
Figure 6 (A) Group data for 16 patients with amyotrophic lateral sclerosis (ALS) and 25 controls showing a reduction in the compound muscle action potential (CMAP) amplitude generated by a submaximal stimulus. (B) Changes in CMAP amplitude for a fixed supramaximal stimulus revealed a reduction in CMAP amplitude after maximum voluntary contraction.
Correlations between membrane excitability and clinical parameters
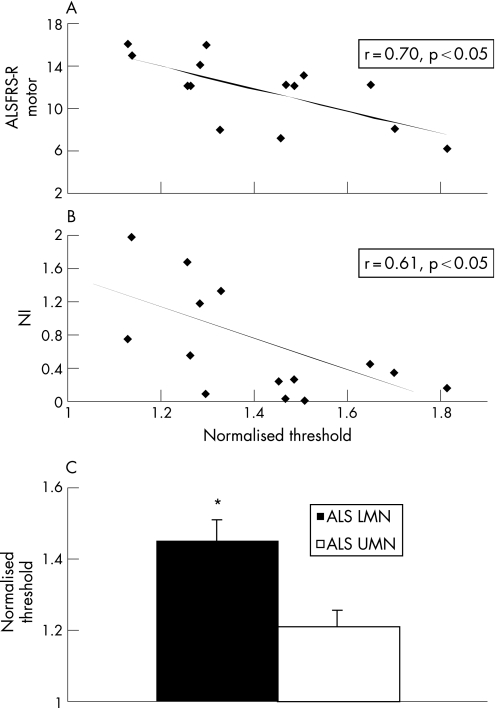
To explore the clinical significance of these activity dependent excitability changes in patients with ALS, correlations were undertaken with clinical and neurophysiological findings. In patients with ALS with limb onset disease, the activity dependent changes in threshold correlated with the ALSFRS‐R gross motor score (fig 7A) and with the neurophysiological index (fig 7B). This suggested that greater activity dependent changes in threshold resulted from an increase in firing rates of the surviving motor axons, possibly in an attempt to compensate for the development of neurogenic weakness.
Figure 7 The increase in normalised threshold correlated with (A) the amyotrophic lateral sclerosis functional rating scale‐revised (ALSFRS‐R) gross motor subscore and (B) the neurophysiological index (NI), a marker of peripheral nerve disease burden. (C) The activity dependent changes in normalised threshold in patients with amyotrophic lateral sclerosis (ALS) with predominantly lower motor neurone (LMN) signs were significantly greater compared with ALS patients with predominantly upper motor neurone (UMN) signs. *p<0.05.
The possibility that greater activity dependent changes resulted from higher firing rates of motor axons was supported by a greater increase in threshold in patients with ALS with predominantly lower motor neurone signs, as indicated by a UMN score of ⩽7 (lower motor neurone normalised threshold 1 ms pulse, 1.44 (0.16); upper motor neurone 1.19 (0.05); controls 1.19 (0.04), 95% CI controls 0.80 to 1.58; p<0.05) (fig 7C). Furthermore, the changes in threshold were greater in patients with ALS with more severe disease, as reflected by CMAP amplitude (threshold in patients with ALS with CMAP <4 mV 44.4 (8.1)%; ALS CMAP >4 mV 30.2 (5.9)%; p = 0.09).
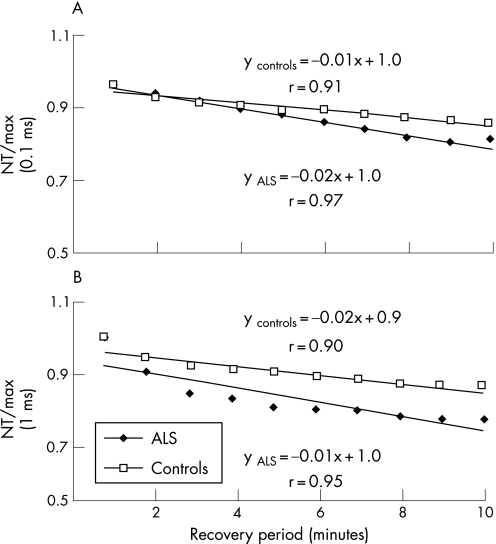
To explore whether greater changes in threshold following activity in patients with ALS reflected alteration in axonal Na+/K+ pump function, recovery of threshold in the early post‐contraction period was compared between patients with ALS and controls. When expressed as a percentage of the maximal change in threshold and then normalised, the slopes of the recovery in threshold were almost identical in the early post‐contraction period for patients with ALS and controls, arguing against any significant difference in Na+/K+ pump function in the ALS patient group (fig 8A, B). Furthermore, the fact that the slope was similar would explain the longer time required for recovery in patients with ALS, given their greater initial threshold increase.
Figure 8 Recovery of threshold for stimuli of (A) 0.1 ms and (B) 1 ms duration for axons from patients with amyotrophic lateral sclerosis (ALS) and controls in the early post‐contraction period. The ratio of normalised threshold recorded during the post‐contraction period compared with the maximal change in threshold immediately following the period of contraction was used as an index of threshold recovery (NT/max). The ratio shows a similar relationship between recovery of threshold for axons from patients with ALS and controls, suggesting that Na+/K+ pump function remains unaffected in patients with ALS.
Discussion
The present study investigated changes in axonal excitability after voluntary contraction in patients with ALS, to further understand the peripheral factors that may contribute to muscle fatigue and weakness in ALS. As a group, the mean total MFI score was 58 (6.4), confirming that fatigue was a common symptom in the present series of patients with ALS. Maximum voluntary contraction induced greater activity dependent changes in threshold in patients with ALS compared with controls. Changes in threshold were associated with an increase in superexcitability, reduction in τSD and submaximal CMAP amplitude, all features consistent with membrane hyperpolarisation.17 While these activity dependent changes were more pronounced in patients with ALS with predominantly lower motor neurone involvement, they were insufficient to induce conduction failure. In total, these findings demonstrate that there is a major contribution of peripheral factors to muscle fatigue, particularly in patients with ALS with predominantly lower motor neurone dysfunction.
Mechanism of activity dependent hyperpolarisation
The electrogenic Na+/K+ pump is integral to the maintenance of normal resting membrane potential and extrudes three Na+ ions for every two K+ ions into the axon, leading to a net deficit of positive charge on the inner aspect of the axonal membrane.46 Activity alters resting membrane potential through effects mediated by the Na+/K+ pump, with membrane depolarisation occurring during MVC resulting from Na+ influx, followed by an increase in Na+/K+ pump activity immediately following contraction that results in membrane hyperpolarisation.17,18,19,20,47 In vitro studies have demonstrated that the post‐tetanic increase in axonal threshold22,47,48 may be abolished following application of ouabain,49 a potent inhibitor of the Na+/K+ pump.19,21
In the present study, there was a greater increase in threshold following voluntary contraction in patients with ALS compared with controls. These changes were accompanied by changes in other excitability parameters indicating membrane hyperpolarisation. Given that threshold changes in patients with ALS as a group were significantly greater compared with controls, a simple explanation for this finding may be that the Na+/K+ pump is overactive. However, as the slopes of threshold recovery were almost identical in patients with ALS and controls (fig 7A, B), Na+/K+ pump overactivity is clearly not the cause. Alternatively, given that the magnitude of the activity dependent hyperpolarisation depends on the impulse load delivered to the axon,17 greater activity dependent changes in threshold may result from higher firing rates of surviving motor units in the setting of neurogenic weakness. Supporting this hypothesis are findings that the increase in threshold correlated with measures of peripheral disease burden and was most evident in patients with ALS with predominantly lower motor neurone involvement. As such, these findings would suggest a major contribution of peripheral factors in increased fatigability in a subgroup of patients with ALS with predominantly lower motor neurone involvement.
These findings are in keeping with a previous study reporting impairment in muscle activation as a mechanism of fatigue in ALS.12 Dysfunction of excitation contraction coupling (ECC) was demonstrated to be the mechanism underlying the failure of muscle activation. Given that impaired ECC in the surviving motor units results in faster firing frequencies,50 and therefore potentially greater activity dependent hyperpolarisation, the findings in the present study would be in keeping with dysfunction of ECC as a mechanism of fatigue in ALS.
Exercise, fatigue and neurodegeneration in ALS
Given that patients with ALS suffer from higher fatigability during physical exertion, the role of exercise in ALS remains an open discussion. Specifically, while some have reported an adverse effect of exercise in terms of precipitating the onset of ALS, others have suggested a beneficial role of physical activity in maintaining muscular conditioning and diminishing symptoms of fatigue.
It has also been suggested that Na+/K+ ATPase dysfunction may be responsible for motor neurone loss in ALS.51 Disruption of resting membrane potential may lead to secondary effects mediated by changes in intracellular Na+, with resultant reverse activation of the Na+–Ca2+ exchanger, leading to intracellular increases in Ca2+ concentration, activation of calcium dependent enzyme systems and neuronal death.52 Of relevance, widespread loss and dysfunction of Na+/K+ pump function has been demonstrated in the superoxide dismutase‐1 ALS mouse model.51 In the present study, the development of activity dependent hyperpolarisation would argue against Na+/K+ pump dysfunction at the axonal level, consistent with previous findings.53 However, the present study does not absolutely dismiss the possibility of Na+/K+ pump dysfunction as a mechanism of neurodegeneration in ALS. Specifically, it could be argued that Na+/K+ pump dysfunction occurred in the axons that had prematurely died, with resultant reduction in CMAP amplitude, and that the surviving axons, which could be assessed in the present study, had either normal pump function or had a stronger reserve capacity of the Na+/K+ pump. As such, in vitro studies assessing axonal pump function would be required to further address this critical issue.
Acknowledgements
SV was awarded the Clinical Fellowship of Motor Neurone Disease Research Institute of Australia (MNDRIA), with funding provided by the Motor Neurone Disease Association of NSW. Grant support was also received from the NSW Ministry for Science and Medical Research Spinal Cord Injury and Related Neurological Conditions Research Grant Program and from the National Health and Medical Research Council of Australia.
Abbreviations
ALS - amyotrophic lateral sclerosis
ALSFRS‐R - amyotrophic lateral sclerosis functional rating scale‐revised
APB - abductor pollicis brevis
CMAP - compound muscle action potential
ECC - excitation contraction coupling
MFI - multidimensional fatigue inventory
MVC - maximum voluntary contraction
τSD - strength duration time constant
UMN - upper motor neurone
Footnotes
Competing interests: None.
References
- 1.Desai J, Swash M.Essentials of diagnosis. London: WB Saunders, 20021–21.
- 2.Kiernan M C. Motor neurone disease: a Pandora's box. Med J Aust 2003178311–312. [DOI] [PubMed] [Google Scholar]
- 3.Rowland L P, Shneider N A. Amyotrophic lateral sclerosis. N Engl J Med 20013441688–1700. [DOI] [PubMed] [Google Scholar]
- 4.Almer G, Vukosavic S, Romero N.et al Inducible nitric oxide synthase up‐regulation in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem 1999722415–2425. [DOI] [PubMed] [Google Scholar]
- 5.Rothstein J D, Jin L, Dykes‐Hoberg M.et al Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci U S A 1993906591–6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trotti D, Rolfs A, Danbolt N C.et al SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter. Nat Neurosci 19992848. [DOI] [PubMed] [Google Scholar]
- 7.Rosen D R, Siddique T, Patterson D.et al Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 199336259–62. [DOI] [PubMed] [Google Scholar]
- 8.Beckman J S, Koppenol W H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 19962711424–1437. [DOI] [PubMed] [Google Scholar]
- 9.Crow J P, Sampson J B, Zhuang Y.et al Decreased zinc affinity of amyotrophic lateral sclerosis‐associated superoxide dismutase mutants leads to enhanced catalysis of tyrosine nitration by peroxynitrite. J Neurochem 1997691936–1944. [DOI] [PubMed] [Google Scholar]
- 10.Andrus P K, Fleck T J, Gurney M E.et al Protein oxidative damage in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem 1998712041–2048. [DOI] [PubMed] [Google Scholar]
- 11.Sanjak M, Brinkmann J, Belden D S.et al Quantitative assessment of motor fatigue in amyotrophic lateral sclerosis. J Neurol Sci 200119155–59. [DOI] [PubMed] [Google Scholar]
- 12.Sharma K R, Kent‐Braun J A, Majumdar S.et al Physiology of fatigue in amyotrophic lateral sclerosis. Neurology 199545733–740. [DOI] [PubMed] [Google Scholar]
- 13.Thomas C K, Zijdewind I. Fatigue of muscles weakened by death of motoneurons. Muscle Nerve 20063321–41. [DOI] [PubMed] [Google Scholar]
- 14.Carpentier A, Duchateau J, Hainaut K. Motor unit behaviour and contractile changes during fatigue in the human first dorsal interosseus. J Physiol (Lond) 2001534903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feasson L, Camdessanche J P, El Mhandi L.et al Fatigue and neuromuscular diseases. Ann Readapt Med Phys 200649375–384. [DOI] [PubMed] [Google Scholar]
- 16.Robbins R A, Simmons Z, Bremer B A.et al Quality of life in ALS is maintained as physical function declines. Neurology 200156442–444. [DOI] [PubMed] [Google Scholar]
- 17.Vagg R, Mogyoros I, Kiernan M C.et al Activity‐dependent hyperpolarization of human motor axons produced by natural activity. J Physiol (Lond) 1998507919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burke D, Kiernan M C, Bostock H. Excitability of human axons. Clin Neurophysiol 20011121575–1585. [DOI] [PubMed] [Google Scholar]
- 19.Bostock H, Grafe P. Activity‐dependent excitability changes in normal and demyelinated rat spinal root axons. J Physiol (Lond) 1985365239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bostock H, Bergmans J. Post‐tetanic excitability changes and ectopic discharges in a human motor axon. Brain 1994117913–928. [DOI] [PubMed] [Google Scholar]
- 21.Morita K, David G, Barrett J N.et al Posttetanic hyperpolarization produced by electrogenic Na(+)‐K+ pump in lizard axons impaled near their motor terminals. J Neurophysiol 1993701874–1884. [DOI] [PubMed] [Google Scholar]
- 22.Kiernan M C, Lin C S, Burke D. Differences in activity‐dependent hyperpolarization in human sensory and motor axons. J Physiol (Lond) 2004558341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cappelen‐Smith C, Kuwabara S, Lin C S.et al Activity‐dependent hyperpolarization and conduction block in chronic inflammatory demyelinating polyneuropathy. Ann Neurol 200048826–832. [PubMed] [Google Scholar]
- 24.Kaji R, Bostock H, Kohara N.et al Activity‐dependent conduction block in multifocal motor neuropathy. Brain 20001231602–1611. [DOI] [PubMed] [Google Scholar]
- 25.Nodera H, Bostock H, Izumi Y.et al Activity‐dependent conduction block in multifocal motor neuropathy: magnetic fatigue test. Neurology 200667280–287. [DOI] [PubMed] [Google Scholar]
- 26.Brooks B R, Miller R G, Swash M.et al El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 20001293–299. [DOI] [PubMed] [Google Scholar]
- 27.Lacomblez L, Bensimon G, Leigh P N.et al Dose‐ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet 19963471425–1431. [DOI] [PubMed] [Google Scholar]
- 28.Zoing M C, Burke D, Pamphlett R.et al Riluzole therapy for motor neurone disease: an early Australian experience (1996–2002). J Clin Neurosci 20061378–83. [DOI] [PubMed] [Google Scholar]
- 29.Kiernan M C. Riluzole: a glimmer of hope in the treatment of motor neurone disease. Early experience confirms that riluzole improves survival and is well tolerated. Med J Aust 2005182319–320. [PubMed] [Google Scholar]
- 30.Smets E M, Garssen B, Bonke B.et al The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 199539315–325. [DOI] [PubMed] [Google Scholar]
- 31.Cedarbaum J M, Stambler N, Malta E.et al The ALSFRS‐R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 199916913–21. [DOI] [PubMed] [Google Scholar]
- 32.Medical Research Council Aid to the examination of the peripheral nervous system. 1 vol. London: Her Majesty's Stationary Office, 1976
- 33.Triggs W J, Menkes D, Onorato J.et al Transcranial magnetic stimulation identifies upper motor neuron involvement in motor neuron disease. Neurology 199953605–611. [DOI] [PubMed] [Google Scholar]
- 34.Vucic S, Howells J, Trevillion L.et al Assessment of cortical excitability using threshold tracking techniques. Muscle Nerve 200633477–486. [DOI] [PubMed] [Google Scholar]
- 35.Vucic S, Kiernan M C. Novel threshold tracking techniques suggest that cortical hyperexcitability is an early feature of motor neuron disease. Brain 20061292436–2446. [DOI] [PubMed] [Google Scholar]
- 36.de Carvalho M, Swash M. Nerve conduction studies in amyotrophic lateral sclerosis. Muscle Nerve 200023344–352. [DOI] [PubMed] [Google Scholar]
- 37.Kiernan M C, Burke D, Andersen K V.et al Multiple measures of axonal excitability: a new approach in clinical testing. Muscle Nerve 200023399–409. [DOI] [PubMed] [Google Scholar]
- 38.Bostock H, Cikurel K, Burke D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve 199821137–158. [DOI] [PubMed] [Google Scholar]
- 39.Weiss G. Sur la possibilité de rendre comparables entre eux les appareils servant l'excitation électrique. Arch Ital Biol 190135413–446. [Google Scholar]
- 40.Mogyoros I, Kiernan M C, Burke D. Strength–duration properties of human peripheral nerve. Brain 1996119439–447. [DOI] [PubMed] [Google Scholar]
- 41.Barrett E F, Barrett J N. Intracellular recording from vertebrate myelinated axons: mechanism of the depolarizing afterpotential. J Physiol (Lond) 1982323117–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.David G, Modney B, Scappaticci K A.et al Electrical and morphological factors influencing the depolarizing after‐potential in rat and lizard myelinated axons. J Physiol (Lond) 1995489141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner M R, Cagnin A, Turkheimer F E.et al Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)‐PK11195 positron emission tomography study. Neurobiol Dis 200415601–609. [DOI] [PubMed] [Google Scholar]
- 44.Mogyoros I, Kiernan M C, Burke D.et al Strength–duration properties of sensory and motor axons in amyotrophic lateral sclerosis. Brain 1998121851–859. [DOI] [PubMed] [Google Scholar]
- 45.Bostock H, Rothwell J C. Latent addition in motor and sensory fibres of human peripheral nerve. J Physiol (Lond) 1997498277–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rakowski R F, Gadsby D C, De Weer P. Stoichiometry and voltage dependence of the sodium pump in voltage‐clamped, internally dialyzed squid giant axon. J Gen Physiol 198993903–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bergmans J.The physiology of single human nerve fibres. Louvain: Vander, 1970
- 48.Kiernan M C, Mogyoros I, Hales J P.et al Excitability changes in human cutaneous afferents induced by prolonged repetitive axonal activity. J Physiol (Lond) 1997500255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaji R, Sumner A J. Ouabain reverses conduction disturbances in single demyelinated nerve fibers. Neurology 1989391364–1368. [DOI] [PubMed] [Google Scholar]
- 50.Vollestad N K, Sejersted O M, Bahr R.et al Motor drive and metabolic responses during repeated submaximal contractions in humans. J Appl Physiol 1988641421–1427. [DOI] [PubMed] [Google Scholar]
- 51.Ellis D Z, Rabe J, Sweadner K J. Global loss of Na,K‐ATPase and its nitric oxide‐mediated regulation in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci 20032343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stys P K, Waxman S G, Ransom B R. Na(+)‐Ca2+ exchanger mediates Ca2+ influx during anoxia in mammalian central nervous system white matter. Ann Neurol 199130375–380. [DOI] [PubMed] [Google Scholar]
- 53.Mogyoros I, Kiernan M C, Burke D.et al Ischemic resistance of cutaneous afferents and motor axons in patients with amyotrophic lateral sclerosis. Muscle Nerve 1998211692–1700. [DOI] [PubMed] [Google Scholar]