Abstract
We describe four families with late onset episodic vertical oscillopsia and progressive gait ataxia. Probands presented between the ages of 40 and 64 years with initial symptoms of episodic vertical oscillopsia and interictal downbeat nystagmus. A mild gait ataxia developed over several years. Triggers included physical exertion, alcohol and caffeine. Patients did not respond to acetazolamide. Genetic screening for episodic ataxia types 1 and 2, and spinocerebellar ataxias 1, 2, 3 and 6 were negative. Using ancestral identity by descent analysis and dense single nucleotide polymorphism (SNP) genotyping throughout the genome, an interval of 28.6 cM (∼14.2 Mb) on chromosome 13q12.11–q13.3, composed of 1259 SNPs, was shared between affected individuals in two of the four families and highlighted a region of suggestive linkage (LOD >2.7).
Oscillopsia is a visual illusion of movement caused by a mismatch in either the gain or the timing of eye movement versus head movement.1 Patients describe seeing the world “oscillate,” “bounce” or “shake.” Oscillopsia can be caused by inadequate compensation of eye movement for head movement, inadequate suppression of the vestibulo‐ocular reflex or by adventitious eye movements. Oscillopsia secondary to cerebellar dysfunction is usually associated with downbeat nystagmus and involves dysfunction of the gaze holding pathways, particularly the flocculonodular lobe.2 Episodic oscillopsia has been described in patients with Arnold Chiari malformations,3 bilateral superior canal dehiscence4 and Tullio phenomenon.5 A family with positional downbeat nystagmus with episodic vertical oscillopsia was described by Kattah and Gujrati.6 Three additional probands who experienced late onset episodic vertical oscillopsia with interictal downbeat nystagmus and a slowly progressive gait ataxia were identified in our database and their clinical features are described here. We used Affymetrix 250K Nsp1 single nucleotide polymorphism (SNP) arrays and a novel ancestral identity by descent (IBD) mapping technique to identify a single haplotype of 28.6 cM (14.2 Mb) on chromosome 13 shared between two of the four families, suggesting a potential interval of linkage. This case series adds to the differential diagnosis of episodic vertical oscillopsia.
Case material
Family 50
A 71‐year‐old woman presented at the age of 62 years with a 6 year history of intermittent vertical oscillopsia and progressive gait imbalance. Her spells were triggered by heat, fatigue and emotional stress and lasted 15–20 min. Interictal examination revealed primary position downbeat nystagmus with gaze evoked and rebound nystagmus. Saccade accuracy was normal but smooth pursuit was impaired. Limb coordination was normal. Gait was wide based; the patient was unable to tandem walk. Episodes were not reduced with 250 mg of acetazolamide twice daily. The patient's mother and maternal aunt developed gait problems in their 50–60s (table 1).
Table 1 Clinical characteristics of the probands and known affected family members.
| Family 50 | Family 71 | Family 158 | Family 396 | |||
|---|---|---|---|---|---|---|
| Sex | F | F | F | M | F | M |
| Age of onset (y) | 56 | 53 | 64 | 53 | 45 | 40 |
| First symptom | Vertical oscillopsia | Vertical oscillopsia | Vertical oscillopsia | Vertical oscillopsia | Vertical oscillopsia | Oscillopsia |
| Episodic | Yes | Yes | Yes | Yes | Yes | Yes |
| Progressive | Mild | Mild | Mild | Mild | Mild | Unknown |
| Duration (min) | 15–20 | 30–60 | 30 | 15–20 | 5 | Unknown |
| Family history | Possibly mother, maternal aunt, maternal grandfather | Sister | Sister | Sister and mother | Brother and mother | None |
| Triggers | Emotional stress, fatigue, physical exertion | Alcohol, physical exertion, bright lights | Alcohol, physical exertion, caffeine, bright lights | Alcohol, exercise, lateral or down gaze | Caffeine, alcohol, exercise, stress | Unknown |
| Response to ACTZ | No | Unknown | Minimal | No | No | Not tried |
| Nystagmus | ||||||
| Gaze evoked | Yes | Yes | Yes | Yes | Yes | No |
| Rebound | Yes | |||||
| Downbeat | Yes* | Yes* | Yes† | Yes* | Yes* | Yes* |
| Upbeat | No | No | No | No | No | Yes |
| Saccades | Normal | Normal | Normal | Hypermetric | Normal | Normal |
| Smooth pursuit | Abnormal | Abnormal | Abnormal | Abnormal | Abnormal | Normal |
ACTZ, acetazolamide.
Ocular motor examinations were performed interictally.
*, on primary position; †, on lateral gaze only.
Family 71
A 62‐year‐old woman presented at the age of 55 years with a 2 year history of episodic vertical oscillopsia lasting 5–10 min triggered by alcohol and exertion. During the episodes, she felt “drunk” and staggered slightly when walking. Her husband noted her eyes “fluttering up and down” during the attacks. Interictal examination showed normal eye movements in the primary position but downbeat nystagmus in all head hanging positions. Coordination and gait were excellent. Follow‐up examination at age 62 years revealed gaze evoked and primary position downbeat nystagmus, a wide based ataxic gait and an inability to tandem walk. Limb coordination was normal. Her spells eventually became continuous. She could not tolerate acetazolamide or 4‐aminopyridine. An older sister had similar signs and symptoms and did not improve with 250 mg of acetazolamide twice daily.
Family 158
A 56‐year‐old man presented with 4 years of episodic vertical oscillopsia triggered by fatigue and progressive interictal gait imbalance. Spells lasted 15–20 min and were associated with mild vertigo and nausea. The symptoms eventually became continuous. Interictal examination revealed downbeat and gaze evoked nystagmus that increased in the head hanging position as well as abnormal smooth pursuit. Saccades were hypermetric but of normal speed. Mild limb incoordination was present in the legs. The patient had difficulty with tandem walking. Episodes were not reduced with 1 g of acetazolamide daily, gabapentin, carbamazepine, propranolol or baclofen. Clonazepam decreased his oscillopsia but could not be tolerated.
The patient's mother developed gait ataxia in her 50s and episodic oscillopsia lasting 30–45 min in her 60s. At age 70 years, she had downbeat, gaze evoked and rebound nystagmus and mild truncal ataxia. At age 87 years, she was dysarthric and required a wheelchair. The proband's sister developed exercise induced vertical oscillopsia and vertigo at the age of 45 years which was controlled with activity moderation. She had mild ataxia and positional downbeat nystagmus which increased after 15 min of running. She did not improve with 1 g of acetazolamide daily. This family was originally described by Kattah and Gujrati in 2005.6
Family 396
A 41‐year‐old man presented with a 1 year history of intermittent vertical oscillopsia which interfered with reading. He denied vertigo or gait imbalance. Examination showed intermittent primary position downbeat nystagmus which increased on lateral gaze. Smooth pursuit was symmetrically impaired but saccades were normal. Coordination and gait examinations were normal. Family history was unknown.
MRIs of all probands were normal. The MRI of the sister of the proband in family 158 was reported to show isolated cerebellar atrophy. All patients were screened for EA1 and EA2 by denaturing high performance liquid chromatography in our laboratory and were commercially screened for spinocerebellar ataxias (SCAs) 1, 2, 3 and 6. These were negative.
Genotyping
DNA was extracted from white blood cells from the six individuals described. These individuals were genotyped using Affymetrix 250K NspI arrays according to the manufacturer's recommended procedures within the UCLA DNA Microarray Facility. Eighty‐nine per cent of the SNPs were called at high confidence in all individuals, resulting in a final set of 190 689 SNPs distributed throughout the genome at a mean spacing of 0.02 cM. These were used for ancestral IBD analysis.
Ancestral IBD analysis
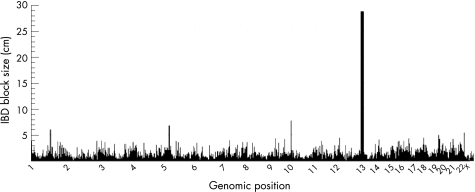
Although the families were not known to be related, we hypothesised that a subset of these families might share ancestors with the risk allele because the phenotype is rare in the general population. All possible pairwise comparisons (n = 6) were performed between affected individuals in different families to search for shared ancestral alleles. By searching for long continuous intervals compatible with a common extended haplotype between the affected individuals, we were able to identify ancestral IBD intervals (B Merriman and SF Nelson, unpublished). This approach is logically similar to genomic mismatch scanning.7 In regions where two affected individuals share an interval of DNA from a common ancestor, the two individuals will have long contiguous intervals of genotypes that are compatible with one allele being shared (no genotypes of AA in one individual and BB in the other individual). We permitted a genotyping error rate of 0.5% and calculated the interval length compatible with a shared interval. Over intervals that are not IBD, the median interval length was 0.14 Mb (SD = 0.8 Mb). Two families (family 158 and 396) shared an unusually large interval of 28.6 cM (∼14.2 Mb) which was composed of 1259 SNPs on chromosome 13q12.11–q13.3 (fig 1). The size of this block is significantly larger than the blocks seen between random pairs in our control database of 300 subjects (p value <10−10). The empiric probability of finding a block this size is 0.002 based on random pairwise comparisons between unrelated individuals (data not shown, n = 10 800 comparisons). These individuals were also shown to have the same genetic background using the Structure Program (http://pritch.bsd.uchicago.edu). This implies that the probands of these two families are more distantly related than third cousins, giving an inferred LOD score of at least 2.7. Amino acid coding regions of genes ATP8A2, WASF3 and KATANL in the region were sequenced based on their high brain expression and roles in cation transport and signal transduction but no mutations were identified. Other candidate genes and regulatory regions in the area are being sequenced.
Figure 1 Genome wide ancestral identity by descent (IBD) mapping analysis between family 158 and family 396. IBD block size (in cM) is plotted against chromosomal position.
Discussion
We have described a syndrome of late onset episodic vertical oscillopsia and slowly progressive gait ataxia. The individuals in family 158 were described in a case report by Kattah and Gujrati,6 but the strong similarities in clinical features seen in this family and those in our database indicate that this is indeed a recognisable syndrome rather than an isolated case. Signs and symptoms indicate midline cerebellar dysfunction, as evidenced by downbeat nystagmus and disproportionately greater truncal compared with limb ataxia. Indeed, autopsy of the proband in family 158 had shown Purkinje loss in the nodulus and flocculus.6 As the patients were seen interictally, we hypothesise that the early spells of vertical oscillopsia were associated with temporary exacerbations of downbeat nystagmus, as noted in family 158 and possibly in family 71. Later, downbeat nystagmus in the primary position became more prominent and the patients complained of more persistent symptoms.
The possible modes of inheritance in these families include sporadic (family 396), autosomal dominant (families 50 and 158) and autosomal recessive (family 71) patterns, suggesting genetic heterogeneity. The identified region of IBD (13q12.11–q13.3) does not overlap with the loci of any of the known SCAs. Age of onset of our patients was higher than observed in the other SCAs that present with pure cerebellar ataxia (SCA 5, 10, 11, 15, 16, 26, 29). These other ataxias do not show episodic features. SCA6, which causes a late onset pure cerebellar ataxia with occasional episodic features,8 was ruled out in all families. This syndrome overlaps with EA2 in terms of triggers and interictal nystagmus. However, all patients tested negative for EA2 and none clearly responded to acetazolamide, which is usually dramatic in EA2. In contrast with our patients, EA2 rarely presents after the age of 20 years.9
A sporadic late onset episodic ataxia syndrome was described by Julien et al in four probands who presented in their 60s with episodic ataxia, slurred speech, diplopia, downbeat nystagmus and interictal progressive ataxia,10 and tested negative for EA2 and SCA6. Although there is stronger evidence of a genetic aetiology in our patients, these are clearly related disorders of episodic cerebellar dysfunction and may represent a spectrum.
Acknowledgements
This project was funded by NIH/NIDCD grant P50DC05224, NIH grant 5U54RR019482 and the Clinical Research Training Fellowship grant from the American Academy of Neurology.
Abbreviations
IBD - identity by descent
SCA - spinocerebellar ataxia
SNP - single nucleotide polymorphism
Footnotes
Funding sources: NIH/NIDCD grant P50DC05224, NIH grant 5U54RR019482 and a Clinical Research Training Fellowship grant from the American Academy of Neurology.
Competing interests: None.
References
- 1.Leigh R J, Zee D.The neurology of eye movements. 4th Edn. Contemporary Neurology Series. Oxford: Oxford University Press, 2006
- 2.Bense S, Best C, Buchholz H ‐ G.et al 18F‐fluorodeoxyglucose hypometabolism in cerebellar tonsil and flocculus in downbeat nystagmus. Neuroreport 200617599–603. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen R, Troost T, Abel L A.et al Intermittent downbeat nystagmus and oscillopsia reversed by suboccipital craniectomy. Neurology 1980301239–1242. [DOI] [PubMed] [Google Scholar]
- 4.Deutschlander A, Strupp M, Jahn K.et al Vertical oscillopsia in bilateral superior canal dehiscence syndrome. Neurology 200462784–787. [DOI] [PubMed] [Google Scholar]
- 5.Cohen H, Allen J R, Congdon S L.et al Oscillopsia and vertical eye movements in Tullio's phenomenon. Arch Otolaryngol Head Neck Surg 1995121459–462. [DOI] [PubMed] [Google Scholar]
- 6.Kattah J C, Gujrati M. Familial positional downbeat nystagmus and cerebellar ataxia: clinical and pathologic findings. Ann NY Acad Sci 20051039540–543. [DOI] [PubMed] [Google Scholar]
- 7.Nelson S F, McCusker J H, Sander M A.et al Genomic mismatch scanning: a new approach to genetic linkage mapping. Nat Genet 1993411–18. [DOI] [PubMed] [Google Scholar]
- 8.Jen J C, Yue Q, Karrim J.et al Spinocerebellar ataxia type 6 with positional vertigo and acetazolamide responsive episodic ataxia. J Neurol Neurosurg Psychiatry 199865565–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jen J C, Kim G W, Baloh R W. Clinical spectrum of episodic ataxia type 2. Neurology 20046217–22. [DOI] [PubMed] [Google Scholar]
- 10.Julien J, Denier C, Ferrer X.et al Sporadic late onset paroxysmal cerebellar ataxia in four unrelated patients: a new disease? J Neurol 2001248209–214. [DOI] [PubMed] [Google Scholar]