Abstract
We describe the clinical course, with special attention to the disturbance of eye movements, of a 29‐year‐old man with chronic ataxic neuropathy with ophthalmoplegia, IgM paraprotein, cold agglutinins and anti‐GD1b disialosyl antibodies (CANOMAD). Using the magnetic search coil technique, we documented convergence during upward saccades and other features suggestive of dorsal midbrain syndrome. Thus, in common with Miller Fisher syndrome, CANOMAD may present with clinical findings implicating involvement of the central nervous system, which contains ganglioside antigens to anti‐GD1b antibodies.
Acute neuropathies may cause ophthalmoplegia, including Miller Fisher syndrome (MFS), which is also characterised by limb and gait ataxia, and the presence of anti‐GQ1b IgG autoantibodies in the serum.1 In MFS, abnormal eye movements often suggest involvement of the central nervous system, mimicking gaze palsies or internuclear ophthalmoplegia.2 Here we report a patient with chronic neuropathy due to anti‐GD1b IgM autoantibodies,3 who presented with disordered eye movements suggesting midbrain disease.
Case report
A 29‐old‐man presented with weakness, difficulty walking and double vision. He had experienced thigh cramps since his late teen years which, in the previous 3 years, had worsened and generalised, involving his arms, legs, chest and neck. For 2 years he also noted slowly increasing imbalance, weakness and intermittent double vision, with horizontal and vertical components. His weakness had progressively spread up his lower extremities to involve his hands. More recently, he experienced burning sensations in his feet and fingers, and urinary urgency, with occasional incontinence. Complete blood count, basic metabolic profile, serum and urine protein electrophoresis, thyroid function tests, vitamin B12 and antinuclear antibody screen were all reported to be normal. His serum creatinine kinase level was elevated at 627 U/l. MRI of the brain, including midbrain, with gadolinium was normal. CSF protein was 86 mg/dl, with no cells. Electromyography indicated a primary neuropathic process. Chronic inflammatory demyelinating polyneuropathy was diagnosed and he was treated with a course of intravenous immunoglobulins and prednisone 60 mg/day. However, his symptoms began to worsen and, when we first evaluated him, he was having difficulty getting out of bed. In addition, he had developed daily headaches, intermittent tachycardia and hyperhidrosis, suggesting autonomic system involvement.
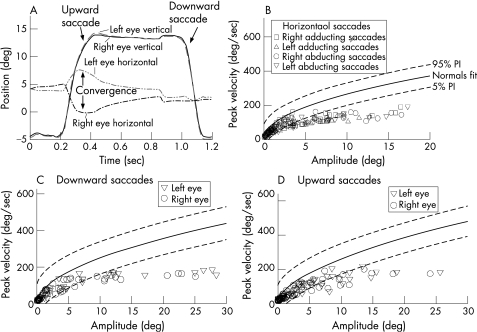
Examination (see video clip; the video clip can be viewed on the J Neurol Neurosurg Psychiatry website at http://www.jnnp.com/supplemental) showed mild, bilateral ptosis and an A pattern exotropia that maximised on down gaze and was almost absent in upgaze. All saccades were slow, and upward saccades were accompanied by convergence. During sustained attempted upgaze, he developed upbeat convergence nystagmus. Smooth pursuit was impaired but the vestibulo‐ocular reflex was normal. His pupils responded better to near stimuli than to light stimuli. We measured his eye movements (magnetic search technique)2 and confirmed that he converged with each upward saccade (fig 1A) and, to a less extent, with horizontal saccades. Saccades in all directions were slow but the peak velocity of each eye was similar (fig 1B–D). Other cranial nerves, including facial muscles, were intact. In his upper and lower limbs, there was symmetrical weakness, worse distally (3‐4/5 MRC scale), with normal strength proximally. He was areflexic; plantar responses were bilaterally flexor. Sensation was impaired for all test modalities, being worse in his lower limbs. Limb ataxia was evident during finger‐to‐nose and heel‐to‐knee‐to‐shin testing. Romberg sign was present, and his gait was wide based, with inability to walk in tandem or on his toes.
Figure 1 Summary of measurements of saccadic eye movements. (A) Time plot of vertical saccades; during the upward saccade, a convergence movement occurs (positive values correspond to upward or rightward eye rotations). (B–D) Plots of peak velocity versus amplitude of horizontal and vertical saccades; 5% and 95% PI indicate prediction intervals for 10 normal subjects previously reported from our laboratory.2 Note that all saccades are slower than controls, but there are no consistent differences between movements of each eye.
Nerve conduction studies indicated axonal sensorimotor peripheral polyneuropathy: sensory and motor conduction signals were low in amplitude, with mild slowing of distal latencies and conduction velocities. No evidence of conduction block was detected. Needle electromyography indicated active and chronic denervation, worse distally. Several other tests were normal: ischaemic forearm testing; mitochondrial function assays on muscle tissue; small bowel biopsy; CT scan of the chest and abdomen; and positron emission tomography body scan. Left deltoid biopsy showed neurogenic atrophy. Sural nerve biopsy showed axonal neuropathy, without inflammation or demyelination. Anti‐GD1b IgM levels were elevated with a titre of 1:6400 (Athena Diagnostics, Worcester, Massachusetts, USA; reference range GD1b IgM <1:800). However, anti‐MAG, anti‐GQ1b and anti‐GM1 were negative.
He was initially treated with plasma exchanges for several weeks, with improved strength, ability to walk and resolution of autonomic symptoms. Thereafter, he received rituximab and monthly intravenous immunoglobulin infusions for 6 months, and subsequently was maintained on monthly intravenous immunoglobulin infusions. Nine months later, his muscle strength and ataxia had improved, with mild residual hand weakness, truncal ataxia and areflexia.
He underwent surgical correction of his strabismus (recession of lateral rectus muscles). Ocular motility examination, 14 weeks later, showed orthotropia and convergence ability; an exophoria persisted. Range of movement was full, and convergence–retraction movements were diminished during vertical saccades. Light–near dissociation of his pupils remained. Pharmacological testing with 1/8% pilocarpine had no effect on pupil size, indicating the absence of denervation supersensitivity.
Discussion
Our patient presented with the syndrome of chronic ataxic neuropathy with ophthalmoplegia IgM paraprotein, cold agglutinins and anti‐GD1b disialosyl antibodies (CANOMAD). His disturbance of eye movements comprised A pattern exotropia and convergence movements with upward saccades, including convergence nystagmus. Thus the clinical presentation (see video clip; the video clip can be viewed on the J Neurol Neurosurg Psychiatry website at http://www.jnnp.com/supplemental) was suggestive of dorsal midbrain syndrome. Light–near dissociation of the pupils without denervation supersensitivity was also suggestive of a central process.
One process by which disease of the ocular motor periphery can mimic central disorders concerns central adaptive mechanisms. This is well documented in myasthenia gravis, when the central nervous system increases innervation in an attempt to overcome peripheral weakness, causing appearances such as saccadic oscillations following injection of edrophonium.2 In MFS, however, there is also evidence of direct involvement of central structures, and an overlap with Bickerstaff's encephalitis.4 Thus central nervous system involvement in MFS may be due to reaction of anti‐GQ1b IgG autoantibodies against ganglioside antigens within the central nervous system.1 In our patient, convergence with upward saccades might be ascribed to his A pattern exotropia. However, when taken with light–near dissociation of the pupil point, convergence nystagmus pointed more to a central process. This possibility has been postulated by Willison and Yuki,1 since the ganglioside for anti‐GD1b antibodies is also present within the central nervous system.
Our patient's age at onset was unusually young, starting in his twenties. His anti‐disialosyl was monoclonal and did not cross react with anti‐GQ1B. Optimal treatment for CANOMAD is not yet established. Rituximab has failed in patients with anti‐ganglioside mediated neuropathy.5 Our patient did not improve with steroids or intravenous immunoglobulin, but did improve during plasma exchange followed by rituximab and intravenous immunoglobulin. Surgical correction of his strabismus improved his ocular motility and visual symptoms. Further studies are needed to attempt to clarify appropriate treatment regimens for this class of neuropathy.
The video clip can be viewed on the J Neurol Neurosurg Psychiatry website at http://www.jnnp.com/supplemental.
Supplementary Material
Acknowledgements
We are grateful to Dr Alok Baghat for referring the patient and to Dr Robert B Daroff for his advice.
Abbreviations
CANOMAD - chronic ataxic neuropathy with ophthalmoplegia, IgM paraprotein, cold agglutinins and anti‐GD1b disialosyl antibodies
MFS - Miller Fisher syndrome
Footnotes
Supported by NIH grant EY06717, the Office of Research and Development, Medical Research Service, Department of Veterans Affairs and the Evenor Armington Fund.
Competing interests: None.
The video clip can be viewed on the J Neurol Neurosurg Psychiatry website at http://www.jnnp.com/supplemental.
References
- 1.Willison H J, Yuki N. Peripheral neuropathies and anti‐glycolipid antibodies. Brain 20021252591–2625. [DOI] [PubMed] [Google Scholar]
- 2.Leigh R J, Zee D S.The neurology of eye movements (book/DVD). 4th Edn. New York: Oxford University Press, 2006
- 3.Willison H J, O'Leary C P, Veitch J.et al The clinical and laboratory features of chronic sensory ataxic neuropathy with anti‐disialosyl IgM antibodies. Brain 20011241968–1977. [DOI] [PubMed] [Google Scholar]
- 4.Yuki N, Wakabayashi K, Yamada M.et al Overlap of Guillain–Barré syndrome and Bickerstaff's brainstem encephalitis. J Neurol Sci 1997145119–121. [DOI] [PubMed] [Google Scholar]
- 5.Rojas‐Garcia R, Tizzano E, Cusco I.et al Chronic neuropathy with IgM anti‐ganglioside antibodies: lack of long term response to rituximab. Neurology 2003611814–1816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.