Abstract
Recombinant plasmids were constructed by fusing either promoter p1 or p2 or both promoters of the rrnB gene of Escherichia coli to a DNA fragment coding for the N-terminal alpha-peptide of beta-galactosidase. These plasmids contained various lengths of the 5'-leader region of rRNA as the 5'-terminal end of the alpha-peptide messenger. In some cases the entire 5'-terminal rRNA-coding sequence was removed, and alpha-peptide synthesis was governed by rac promoters formed by fusion of rrnBp2 and lac promoters. By measuring the level of alpha peptide, conclusions could be drawn about the activities of the promoters under various physiological conditions. It was found that the rate of transcription starting from promoter p1 or p2 might vary more than 10-fold during the growth cycle, showing a sharp maximum during outgrowth from the stationary phase into exponential growth or during nutritional shift-up. The target sequence of this regulation was localized to the leader region of the rrnB gene.
Full text
PDF


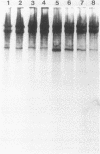
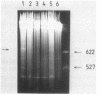
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boros I., Kiss A., Sain B., Somlyai G., Venetianer P. Cloning of the promoters of an Escherichia coli rRNA gene. New experimental system to study the regulation of rRNA transcription. Gene. 1983 May-Jun;22(2-3):191–201. doi: 10.1016/0378-1119(83)90103-8. [DOI] [PubMed] [Google Scholar]
- Boros I., Lukacsovich T., Baliko G., Venetianer P. Expression vectors based on the rac fusion promoter. Gene. 1986;42(1):97–100. doi: 10.1016/0378-1119(86)90154-x. [DOI] [PubMed] [Google Scholar]
- Bremer H., Berry L., Dennis P. P. Regulation of ribonucleic acid synthesis in Escherichia coli B-r: an analysis of a shift-up. II. Fraction of RNA polymerase engaged in the synthesis of stable RNA at different steady-state growth rates. J Mol Biol. 1973 Mar 25;75(1):161–179. doi: 10.1016/0022-2836(73)90536-6. [DOI] [PubMed] [Google Scholar]
- Ginsburg D., Steitz J. A. The 30 S ribosomal precursor RNA from Escherichia coli. A primary transcript containing 23 S, 16 S, and 5 S sequences. J Biol Chem. 1975 Jul 25;250(14):5647–5654. [PubMed] [Google Scholar]
- Glaser G., Sarmientos P., Cashel M. Functional interrelationship between two tandem E. coli ribosomal RNA promoters. Nature. 1983 Mar 3;302(5903):74–76. doi: 10.1038/302074a0. [DOI] [PubMed] [Google Scholar]
- Gourse R. L., Stark M. J., Dahlberg A. E. Regions of DNA involved in the stringent control of plasmid-encoded rRNA in vivo. Cell. 1983 Apr;32(4):1347–1354. doi: 10.1016/0092-8674(83)90315-x. [DOI] [PubMed] [Google Scholar]
- Gourse R. L., de Boer H. A., Nomura M. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell. 1986 Jan 17;44(1):197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- Holben W. E., Morgan E. A. Antitermination of transcription from an Escherichia coli ribosomal RNA promoter. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6789–6793. doi: 10.1073/pnas.81.21.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holben W. E., Prasad S. M., Morgan E. A. Antitermination by both the promoter and the leader regions of an Escherichia coli ribosomal RNA operon. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5073–5077. doi: 10.1073/pnas.82.15.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajitani M., Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase. Differential stringent control of the multiple promoters from ribosomal RNA and protein operons. J Biol Chem. 1984 Feb 10;259(3):1951–1957. [PubMed] [Google Scholar]
- Li S. C., Squires C. L., Squires C. Antitermination of E. coli rRNA transcription is caused by a control region segment containing lambda nut-like sequences. Cell. 1984 Oct;38(3):851–860. doi: 10.1016/0092-8674(84)90280-0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Muto A. Control of ribosomal RNA synthesis in Escherichia coli. IV. Frequency of transcription of ribosomal RNA genes as a function of growth rate. Mol Gen Genet. 1978 Aug 4;164(1):39–44. doi: 10.1007/BF00267596. [DOI] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Sarmientos P., Cashel M. Carbon starvation and growth rate-dependent regulation of the Escherichia coli ribosomal RNA promoters: differential control of dual promoters. Proc Natl Acad Sci U S A. 1983 Nov;80(22):7010–7013. doi: 10.1073/pnas.80.22.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmientos P., Sylvester J. E., Contente S., Cashel M. Differential stringent control of the tandem E. coli ribosomal RNA promoters from the rrnA operon expressed in vivo in multicopy plasmids. Cell. 1983 Apr;32(4):1337–1346. doi: 10.1016/0092-8674(83)90314-8. [DOI] [PubMed] [Google Scholar]
- Schleif R. Control of production of ribosomal protein. J Mol Biol. 1967 Jul 14;27(1):41–55. doi: 10.1016/0022-2836(67)90350-6. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]