Abstract
Neurology encompasses all aspects of medicine and surgery, but is closer to orthopaedic surgery than many other specialities. Both neurological deficits and bone disorders lead to locomotor system abnormalities, joint complications and limb problems. The main neurological conditions that require the attention of an orthopaedic surgeon are disorders that affect the lower motor neurones. The most common disorders in this group include neuromuscular disorders and traumatic peripheral nerve lesions. Upper motor neurone disorders such as cerebral palsy and stroke are also frequently seen and discussed, as are chronic conditions such as poliomyelitis. The management of these neurological problems is often coordinated in the neurology clinic, and this group, probably more than any other, requires a multidisciplinary team approach.
In this review, we discuss the causes and diagnostic approaches, management, rehabilitation and orthopaedic treatment of these conditions, with the exception of the peripheral nerve injuries, which require a separate review.
Diagnosis
Neurological investigations to obtain an accurate diagnosis are essential in the management of each patient. Knowing the natural history of a disorder allows insight into future rehabilitation and treatment needs.1 Before the involvement of the orthopaedic and rehabilitation teams, patients will have been diagnostically investigated with routine blood tests, radiological and electrodiagnostic investigations, and in some cases invasive procedures such as muscle or nerve biopsies. Genetic testing is now widespread, and a positive result can eliminate the need for invasive procedures.2
Functional assessment
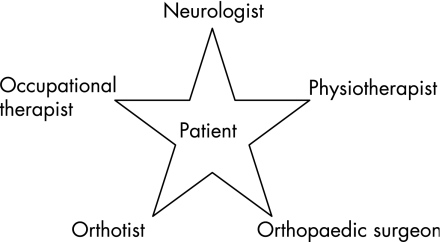
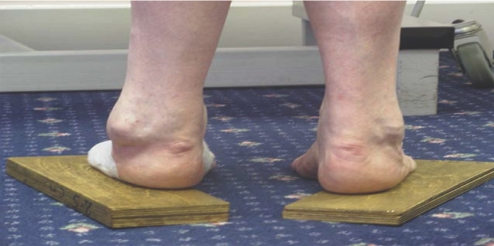
A functional assessment requires the examiner to look, feel and move the patients and their limbs (fig 1). Other members of the multidisciplinary team are essential in this assessment to identify the patients' needs and plan treatment3 (fig 2), and several areas in the assessment should be considered.4
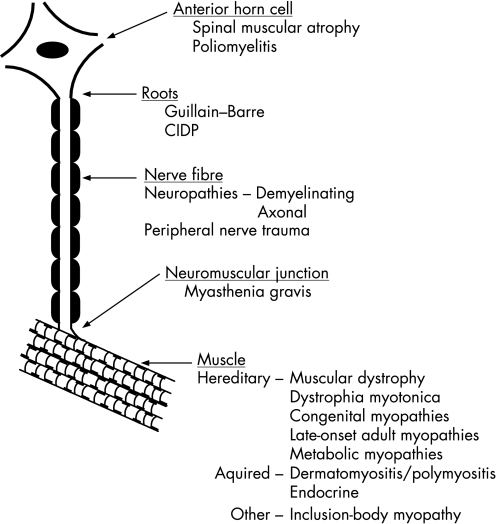
Figure 1 Disorders of the lower motor neurone. CIDP, chronic inflammatory demyelinating polyneuropathy.
Figure 2 Treatment of patients with neuromuscular disorders requires a multidisciplinary team and a neurologist with a special interest in these disorders to coordinate this care. During annual reviews, each aspect of the care should be discussed. Many patients will require additional input from others such as a social worker, chiropractor, dietician and psychologist.
Gait and posture
Typical recognised patterns of neuromuscular disorders include a spastic gait, drop foot gait, high steppage gait, waddling and ataxic gait.
Deformity
When muscles are weak, the joint is unstable and the limb floppy and flail; this is balanced paralysis. Unbalanced paralysis occurs when one group of muscles is relatively weak compared with its antagonist pull; the deformity may be initially correctable, but may become fixed with time.
Muscle weakness
This may be due to upper or lower motor neurone lesions or muscle disorders.
Spasticty and contractures
Spasticity is a frequent finding in patients with upper motor neurone lesions. When the appropriate stretching is not carried out in patients with spasticity, muscle shortening and contractures can occur. This can also occur in patients with lower motor neurone lesions and certain muscle disorders (fig 1). Spasticity can be painful in itself, and can interfere considerably with routine function and daily activities. Consideration should be given to management of specific spasticity rather than to the effects it induces. Considerable advances have been made in both local and systemic pharmaceutical management.
Sensory problems
These can often be the main symptom with pain, and can lead to pressure sores, ulcers and neuropathic (Charcot's) joints.
Autonomic functions
These can often be extremely disabling, with abnormalities of blood pressure, sphincter control and sweating.
Principles of treatment
The main areas of treatment can be applied to long‐term management of most neurological conditions, and these steps should be followed with minor variations according to the disorder5 (box 1). Self‐management should be seen as a long‐term investment and continually encouraged. Stretching and exercise are best advised by the physiotherapist, with an individual plan of exercise drawn up. Physiotherapy and occupational therapy are usually performed regularly on an outpatient basis or, if a burst of intensive therapy is required, they can be performed on an inpatient basis in a rehabilitation unit. Patients may also require specialist services such as their local wheelchair clinic.6,7
Traditional orthoses used to be cumbersome, large, unattractive and often quite uncomfortable to wear. Modern orthoses make use of advances in material technology and are lightweight, flexible, often unobtrusive and comfortable supports that can improve a patient's quality of life.7 Depending on the neurological disorder, patients have different orthotic needs, and these are best assessed by a neurologist, a physiotherapist and an orthotist working together. An orthotic assessment often provides alternatives for intervention, and liaison between an orthotist and a therapist may optimise interventions between providing stability and maximising function. All orthoses should be fitted by the orthotist and physiotherapist; when starting with a new orthosis, patients may need careful physiotherapy review and repeat orthotic follow‐up if there is discomfort or change in presentation. Orthoses are often used to change alignments, which may give the patient and therapist access to more normal movement and muscle function than previously. It is not uncommon for patients to have secondary weakness due to muscle disuse from poor alignment. By combining orthotic and physiotherapy interventions, it may be possible to improve strength and function. However, it should also be recognised that some presentations of poor alignment are necessary compensations, which, if corrected, may lead to reduced stability and loss of function.
The orthopaedic management is discussed in detail in sections related to each particular disorder.8,9
Neuromuscular disorders
Tremendous advances have been made over the past 15 years in understanding the genetic basis of neuromuscular disorders.10 In disorders such as the muscular dystrophies, not only have many genes been localised but also the biochemical basis of the disorders is beginning to be understood (tables 1 and 2).2,11 There is no cure for most of these conditions, but much can be implemented to improve patients' quality of life (box 1).
Table 1 Classification of muscle disease.
| Genetic muscle disease |
|---|
| The muscular dystrophies |
| Dystrophinopathies (Duchenne's/Becker's) |
| Facioscapulohumeral muscular dystrophy |
| Emery–Dreifuss muscular dystrophy |
| Limb girdle muscular dystrophy |
| Myotonic dystrophy |
| Metabolic myopathies |
| Glycogenolytic myopathies (eg, McArdle's disease) |
| Lipid storage myopathies (eg, carnitine palmitoyltransferase II deficiency) |
| Mitochondrial myopathies |
| Skeletal muscle ion‐channel disorders |
| Periodic paralysis disorders |
| Myotonia congenital |
| Congenital myopathies |
| Nemaline myopathy |
| Central core disease |
| Other genetic myopathies |
| Hereditary inclusion‐body myositis |
| Cardiomyopathy‐associated myopathy |
| Distal myopathies |
| Myopathy associated with tubular aggregates or arrays |
| Hereditary cytoplasmic body myopathies |
| Acquired muscle disease |
|---|
| Inflammatory muscle disease |
| Dermatomyositis/polymyositis |
| Inclusion‐body myositis |
| Endocrine myopathies |
| Thyrotoxicosis |
| Cushing's disease |
| Drug‐induced and toxic myopathies |
| Corticosteroids |
| Lipid‐lowering drugs (3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitors) |
| Alcohol |
Table 2 Classification of the major muscular dystrophies.
| Disease | Locus | Protein |
|---|---|---|
| X linked | ||
| Duchenne–Becker | Xp21 | Dystrophin |
| Emery–Dreifuss | Xp28 | Emerin |
| Barth | Xp28 | Tafazzins |
| Danon's disease | Xq24 | LAMP‐2 |
| Autosomal dominant (AD) | ||
| Myotonic dystrophy (DM1) | 19q | Myotonin |
| Myotonic dystrophy (DM2) | 3q21 | ZNF9 |
| FSH dystrophy | 4q deletion | Many proteins |
| LGMD 1A | 5q | Myotilin |
| LGMD 1B | 1q11–12 | Nuclear Lamin |
| LGMD 1C | 3p25 | Caveolin‐3 |
| Emery–Dreifuss | 1q21 | Lamin A/C |
| Bethlem | 21q22 and 2q27 | Collagen VI |
| Central core disease | 19q13 | Ryanodine receptor |
| Myofibrillar (desmin storage) | Several | Several |
| Oculopharyngeal | 14q11 | PABP2 |
| Autosomal recessive (AR) | ||
| LGMD 2A | 15q | Calpain |
| LGMD 2B | 2q | Dysferlin |
| LGMD 2C | 13q | γ‐Sarcoglycan |
| LGMD 2D | 17q | α‐Sarcoglycan |
| LGMD 2E | 4q | β‐Sarcoglycan |
| LGMD 2F | 5q | δ‐Sarcoglycan |
| LGMD 2G | 17q11–12 | Telethonin |
| LGMD 2H | 9q31–q33 | TRIM32 |
| LGMD 2I | 19q33.2 | FKRP1 |
| LGMD 2J | 2q31 | Titin |
| LGMD 2K | 9q34 | POMT1 |
| Congenital dystrophies | ||
| Congenital muscular (AR) | 6q | Merosin |
| Fukuyama disease (AR) | 9q13 | Fukutin |
| Congenital myopathies | ||
| Nemaline rod disease | 1q22 | Topomyosin |
| Myotubular myopathy | Xq26 | MTM1 |
Orthopaedic treatment has aimed at preventing the progression of deformities and providing stability to the skeletal system to improve the quality of life for children and adults.8 Numerous surgeons have reported case series showing an improvement in their patients' activities of daily living. Louis et al12 reported 34 surgical procedures performed to improve sitting posture, care and comfort in patients with various neuromuscular disorders, and all patients reported marked improvements in function. The role of the orthopaedic surgeon in achieving these goals includes prescribing orthoses, preventing or correcting joint contractures, and maintaining appropriate standing and sitting postures.7
The muscular dystrophies
These are a group of hereditary disorders of the skeletal muscle that produce progressive degeneration of the skeletal muscle and associated weakness. The X linked dystrophies such as Duchenne's and Becker's muscular dystrophy (DMD and BMD) are the most common dystrophies encountered in clinical practice (table 2).2,9,13,14 Patients with muscular dystrophy should be treated using the standard rehabilitation principles (box 1). There is a fairly well recognised orthopaedic and orthotic protocol in the management of DMD because of its relatively predictable prognosis and the compensations patients adopt to maintain stability. Patients also have respiratory insufficiency and cardiac abnormalities; these problems should be screened in parallel. They differ in severity according to the diagnosis, as described in the workshop report of Bushby et al.15 Patients with DMD should have an echocardiogram and ECG at diagnosis, before any surgery, every 2 years up to the age of 10 years and annually after the age of 10 years. Female DMD and BMD carriers require similar but less frequent screening.15
Box 1 Principles of treatment
-
Self‐management
-
-
Stretching
-
-
Foot care
-
-
Exercise
-
-
Diet
-
-
-
Physical treatment
-
-
Physiotherapy: active and passive
-
-
Orthotics
-
-
Occupational therapy
-
-
-
Orthopaedic surgery
-
-
Tailored to the individual patient
-
-
Restoring function when possible
-
-
Fracture management
-
-
Management of contractures
-
-
Posture, general care and seating systems
-
-
Orthopaedic treatment
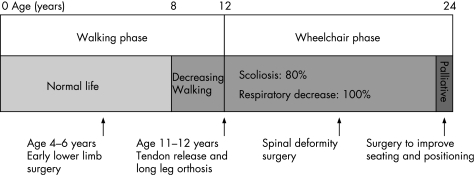
The major goal of early treatment is to maintain functional ambulation as long as possible. Patients with DMD are susceptible to the development of contractures and scoliosis.16 Stretching exercises and nightly bracing can help to prevent the contractures from becoming severe. At the age of 8–15 years, children with DMD have a sensation of locking of the knees. It is easier to keep children with DMD walking than to induce walking once they have stopped, therefore, contractures should be treated early to maintain ambulation, because, if it is maintained even for 1–2 years, it considerably benefits these children and delays the inevitable development of scoliosis and kyphosis17,18 (fig 3).
Figure 3 Evolving phases and features of Duchenne's muscular dystrophy.
Three approaches are usually used to correct lower extremity contractures:
Ambulatory approach (early lower limb surgery): The goal here is to correct any contractures while the patient is still ambulatory. This is often needed early, between 4 and 6 years at the initial appearance of contractures, at the plateau of muscle strength or when there is difficulty maintaining an upright posture with the feet together.19 Rideau19 suggested a three‐step approach to correct early contractures, consisting of bilateral tenotomy of the superficial hip flexors, aponeurectomy of the iliotibial band, and subcutaneous tenotomies of the knee and foot.
Rehabilitative approach: Surgery is performed at or just after the patient has lost the ability to walk, but with the intention that walking will resume. Walking and standing can be extended for a few years by the use of long leg orthoses in boys with DMD who reach the critical phase of loss of independent walking ability.20 Before the use of most orthoses, surgery is required to release the Achilles tendon alone or with superficial hip and medial knee flexors. The mean prolongation of walking was significant at 3.9 years in a series of 59 patients.21
Palliative approach: This is used for treating contractures that interfere with shoe wear and comfortable wheelchair positioning. Even when a boy is too weak to walk, he should continue to stand using a standing frame or a stand‐up wheelchair, such as the Levo and Crest.
Scoliosis
Usually by the age of 12–13 years, most children with DMD can no longer walk, and the progressive spinal deformity becomes the major problem, affecting sitting and causing breathing problems. Three patterns of spinal deformity are seen: kyphoscoliosis with collapse of the spine (the most common), lordoscoliosis and hyperlordosis with rigid spine. Conservative treatment is not effective. Bracing and wheelchair support may slow the progression but spinal fusion is ultimately required.
Posterior spinal fusion with segmental instrumentation and arthrodesis is the operation of choice.22 Most surgeons recommend early surgery at the onset of the scoliosis when the curve is only 10–20°, but with modern anaesthetic procedures such as tracheotomy, patients presenting late or with respiratory problems can still be operated on with benefit. Fusion of the entire thoracolumbar spine is carried out, extending from the pelvis to the proximal thoracic spine. Facet‐joint arthrodesis is performed at every level with autologous or allograft bone grafting.16
Other types of muscular dystrophy and myopathies
The rehabilitative and surgical procedures can be applied to other types of muscular dystrophy such as BMD and other types of myopathy, although they are usually less severe and require later intervention. Neck hyperextension is common in myopathies and is a late complication of DMD. Giannini et al23 devised a technique that corrects neck hyperextension by opening the interspinous spaces from C2–C7 using a capsulotomy. The correction is stabilised with bone grafts fixed to the spinous processes.
In some types of muscular dystrophy (limb‐girdle and facioscapulohumeral in particular), the inability to functionally flex and abduct the shoulder is usually treated by stabilisation of the scapula with scapulothoracic arthrodesis.9,18
Peripheral neuropathy
Disorders of the peripheral nerves may affect motor, sensory and autonomic nerves. These disorders may be classified as acute or chronic, and split according to their distribution into mononeuropathies, multiple mononeuropathy and polyneuropathy. The treatment and rehabilitation of patients with peripheral neuropathy is directed by the type, patient age and disease severity. Although specific treatment is available for some types of chronic and acute neuropathies, there is often residual impairment. Most neuropathies are untreatable, meaning that rehabilitative practice and surgical procedures are important in long‐term management.2,24
The management of these patients mainly aims to tackle three aspects: weakness, sensory loss and pain.25 In both acute and chronic neuropathies, self‐management and physical treatment are important to maintain to increase power as well as to avoid contractures. Functional splinting can restore function to weak upper limbs, foot drop can be treated by positional procedures, and ankle foot orthoses will correct abnormal gait and help prevent falls. The loss of sensation carries the risk of unnoticed trauma to the skin, with the danger of neuropathic ulceration and arthropathy. Patients must be educated to examine their feet, and have regular chiropody and orthotic assessments. It is important to involve the orthopaedic surgeon early for patients with abnormal feet to plan their future needs.
Leprosy is probably still the most common worldwide cause of neuropathy, where the main treatment options are chemotherapy; some patients may benefit from tendon transfers or nerve grafts.
Diabetic neuropathy is the most common cause of neuropathy in the Western world. The most common management problem is the diabetic foot, where treatment is aimed at skin care, and maintaining tissue integrity. Considerable resources and advances have been made to minimise amputations by optimising orthotic intervention. Orthopaedic management of this group includes the treatment and prevention of ulcers and/or infection and the management of neuropathic joints. Ulcers in neuropathic feet develop because of associated ischaemia, abnormal loading/shear (due to foot deformity or tight Achilles tendons), inappropriate shoe wear or trauma. All patients with diabetic feet (especially those who cannot feel a 10 g Semmes–Weinstein monofilament) should be assessed regularly and associated factors identified and treated. Surgery may include lengthening of the Achilles tendon to reduce forefoot loading, debridement of ulcers, total contact casting of the foot to equalise pressure distribution, removal of necrotic bone and revascularisation procedures.
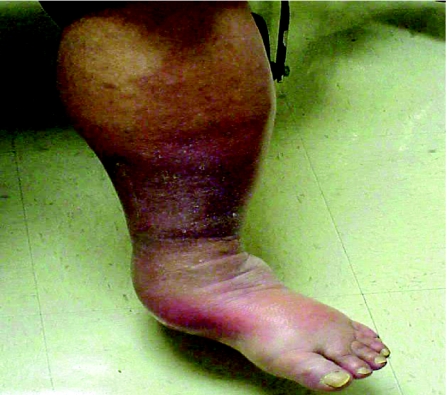
Charcot neuropathic joints (fig 4) are wrongly thought to be painless; they are relatively less painful, but patients do require analgesics. The notion that Charcot joints are painless also leads to failure to diagnose Charcot joints in their early stage when inflammation (pain, swelling, heat and redness) is present. Resolution of the inflammatory features with elevation is a simple test that helps differentiate an acute Charcot joint from other conditions, such as cellulitis, osteomyelitis, or deep vein thrombosis; bone scans, MRI scans or bone biopsy are not necessary. Early recognition and appropriate offloading of weight prevents deformity; in patients who present in the late stages with a deformed or unstable foot, a fusion of appropriate joints may provide a stable plantigrade foot.
Inflammatory neuropathies, such as Guillain–Barré neuropathy, are relatively common worldwide, but they usually respond to intravenous immunoglobulin treatment. Many of these patients have residual weakness and limb problems, some require orthopaedic treatment which are usually tendon transfers. The main group of neuropathies that require long‐term rehabilitative management and orthopaedic treatment are the chronic degenerative neuropathies, where by far the most common groups are genetic.
Hereditary neuropathy
In everyday clinical practice, it is common to encounter patients with peripheral neuropathy of an inherited nature (table 3). Charcot–Marie–Tooth disease (CMT) is the most common inherited neuromuscular disorder, with an estimated overall prevalence of at least 1 in 5000.26 Déjèrine–Sottas disease and congenital hypomyelination are rare early‐onset neuropathies that need to be distinguished from other neuropathies of the paediatric age, as they are severe.
Table 3 Classification of inherited neuropathies.
| 1. Neuropathies in which the neuropathy is the sole or primary part of the disease Charcot–Marie–Tooth | |
| Hereditary neuropathy with liability to pressure palsies | |
| Hereditary sensory and autonomic neuropathies | |
| Hereditary neuralgic amyotrophy | |
| Distal hereditary motor neuronopathies (distal hereditary motor neuropathy or distal spinal muscular atrophy) | |
| 2. Neuropathies in which the neuropathy is a part of a more widespread or multisystem disorder | |
| Familial amyloid polyneuropathies | Transthyretin |
| Apolipoprotein A1 | |
| Gelsolin | |
| Disturbance of metabolism | Leucodystrophies |
| Lipoprotein deficiencies | |
| Phytanic acid storage diseases | |
| α‐Galactosidase deficiency | |
| Cholestanolosis | |
| Sphingomyelin lipidoses | |
| Porphyria | Acute intermittent |
| Hereditary coproporphyria | |
| Variegate | |
| γ‐Aminolaevulinic acid dehydratase deficiency | |
| Disorders of DNA function | Xeroderma pigmentosa |
| Ataxia telangiectasia | |
| Cockayne's syndrome | |
| Mitochondrial disease neuropathies | Various |
| Neuropathy and hereditary ataxia | Friedreich's ataxia |
| Spinocerebellar ataxia | |
| Miscellaneous | Giant axonal neuropathy |
| Neuroacanthocytosis | |
| Chediak–Higashi disease | |
| Allgrove's syndrome | |
CMT is characterised by wasting and weakness of distal limb muscles, usually with distal sensory loss, skeletal deformities (eg, pes cavus), and decrease or absence of tendon reflexes. Disease onset usually occurs during the first or second decade of life and the course is slowly progressive, but the patient's life span is normal. CMT is clinically and genetically heterogeneous. The most common type of CMT is 1A, which is the cause in about 70% of patients. There is an increasing number of genes being identified in CMT and a genetic diagnosis can now be obtained in most pedigrees.2,24
CMT is also the most common neuromuscular cause of foot deformity in children, and occult CMT is frequently the cause of abnormal feet in adults.8 Hand deformities tend to occur in adults, but can also be found in severe childhood neuropathy (box 2).
Box 2 Foot and upper limb problems in Charcot–Marie–Tooth disease
Common foot problems
Foot drop
Very high arches (pes cavus)
Claw toes
Weak ankles
Corns and calluses
Ulcer and sores
Common hand/upper limb problems
Claw fist
Weak wasted hands
Opposition weakness
Pinch weakness
Strain and tightness in shoulder and neck (as muscles in the upper arm try to compensate for loss of hand strength)
The foot abnormality is usually a cavovarus deformity, which is a complex deformity of the forefoot and hindfoot.27 The hindfoot varus deformity is usually secondary to the forefoot pronation, and early orthotic treatment and physiotherapy may delay the onset of fixed hindfoot deformities. Many other foot deformities can arise, because many patients present late. Charcot's joints are unusual in CMT but more often found in patients with HSAN. The weakness of small foot muscles and ankle dorsiflexors with relative preservation of the calf muscles results in dorsiflexion of the proximal phalanx on the metatarsal head, plantar flexion of the distal phalanges, and shortening with secondary contracture of the longitudinal foot arch and varus deformity of the hindfoot. Clawing of the toes, pes cavus, calluses and an inability to find properly fitting shoes are the eventual results of these conditions.
Figure 4 Charcot's foot.
Every patient with CMT with foot problems should have careful clinical and radiological evaluation. This includes Coleman's block test (fig 5) and plain x rays of the foot.28 Initially, the general principles of rehabilitation should be used (box 1). Orthopaedic surgery is carried out only when non‐operative treatment has repeatedly been unsuccessful. Treatment is determined by age of the patient, and the cause and severity of the deformity. Surgical procedures are usually staged and require meticulous aftercare with casting after most operations.28 It is now recognised that arthrodeses procedures without balancing the foot do not achieve good results. Modern treatment is aimed at placing the hindfoot under the heel by means of a calcaneal osteotomy, placing the foot on the ground by means of, for example, metatarsal osteotomies, and balancing the foot by means of appropriate muscle transfers. Arthrodeses are only performed when degenerative changes are present.
Figure 5 Coleman's block test.
Surgical procedures are of three types29,30,31,32,33,34,35,36,37,38,39,40,41:
Soft tissue: plantar fascia release, tendon release, lengthening or tendon transfer
Osteotomy: metatarsal, mid‐foot, calcaneal
Joint stabilising: arthrodesis.
Soft‐tissue releases in children can delay the need for extensive reconstruction. These should be carried out early and aggressively when the hindfoot is flexible. Even in young patients with a fixed hindfoot deformity, limited soft‐tissue release combined with a first metatarsal or calcaneal osteotomy or both can provide a satisfactory functional outcome. The triple arthrodesis is left as a salvage procedure when other methods have not worked.42 Wukich and Bowen43 reported that only 14% of patients with CMT required triple arthrodeses, and they carried out soft‐tissue procedures and osteotomies in the skeletally immature feet and for those with less deformity.
Flexible claw‐toe deformity is usually corrected without additional surgery when the mid‐foot deformity is corrected.9 For severe weakness, a number of procedures transferring tendons or fusing joints can be used. Scoliosis and kyphosis are often seen in CMT, but are usually mild and require only observation. In some of the CMT variants such as Déjèrine–Sottas disease, spinal curvature can be severe, and if conservative management of exercises and bracing are not enough then operative treatment is needed.44
Friedreich's and spinocerebellar ataxias
The spinocerebellar ataxias are a clinically and genetically heterogeneous group. The conditions are clinically linked by cerebellar degeneration but differentiated by their additional clinical features and the results of genetic testing. Like many other neurological disorders, a multidisciplinary approach is essential to patient care using medical and surgical treatment.45
The most common type is Friedreich's ataxia, which is an autosomal recessive condition characterised by spinocerebellar degeneration, peripheral neuropathy, pes cavus, kyphoscoliosis, cardiac and visual problems. In these patients, the general principles of neurorehabilitation should be applied. Additional problems in patients with ataxia include difficulties with communication, difficulty in eating due to the ataxia and resultant weight loss, and mood and behaviour problems.
The orthopaedic surgeon is frequently involved in the correction of spinal deformities where the management is similar to that of idiopathic scoliosis.44 The foot deformities are mainly cavovarus and equinovarus. These are managed in a manner similar to patients with CMT, although some procedures may need to be carried out under local anaesthesia or with a tracheotomy owing to the cardiac risks.46 In Friedreich's ataxia, function and mobility were improved after surgery as rated by the Barthel Index and functional independence measure, suggesting aggressive management of these disorders to improve quality of life.45 Cardiac involvement is a major cause of mortality in Friedreich's ataxia, affecting two thirds of patients, and should be defined by echocardiography as well as ECG at diagnosis, before any procedure; patients should have a minimum of once‐yearly ECG and echocardiography monitoring.47 All patients should be referred to a cardiologist, especially as there are many trials of emerging therapeutic treatment, such as idebadone, that may help cardiac function.48
Spinal muscular atrophy and hereditary motor neuropathy
Spinal muscular atrophy is a recessively inherited condition of the anterior horn cells in the spinal cord, with a frequency of 1 in 10 000 births.10 Clinically, patients have severe proximal limb weakness more than distal limb weakness, hypotonia, areflexia, fine tremor, respiratory problems and cardiac problems, but normal sensation. Type I (Werdnig–Hoffman disease) is the most severe, where children are never able to sit independently; type II (Kugelberg–Welander disease) where they can sit up but cannot walk; type III, where they have limited walking; and type IV, where they can run but develop weakness later in life. Orthopaedic treatment is frequently required for hip and spine problems, and sometimes for fractures and contractures. As many patients are non‐ambulatory, only stable sitting is essential and proximal femoral varus derotational osteotomy can provide this. Coxa vara deformity is frequent, and osteotomies and trochanter transfer are used for walking patients.49 Progressive scoliosis eventually affects nearly 100% of patients and should be managed early before the curve becomes too severe.44,50 Bracing is indicated in the early years, and spinal stabilisation with long posterior fusion (longer and higher than idiopathic scoliosis) is required in most adults. Distal spinal muscular atrophy or hereditary motor neuropathy is a rare but chronic disorder with a normal life span. These patients frequently require lower limb tendon transfers and orthotic management.49
Hereditary sensory and autonomic neuropathy
Hereditary sensory and autonomic neuropathy is a progressive neuropathy that primarily affects the sensory nerves but can affect the motor nerves later in life.51 There is a number of clinical and genetic variations of hereditary sensory and autonomic neuropathy (table 4).52 Patients usually present with numbness, paraesthesias, pain and neuropathic ulcers, although some of the classification groups such as Riley–Day syndrome are more widespread.53 The ulcers are typically slow to heal and often become infected (fig 6). Many patients require debridement, skin grafting and amputations to improve healing, preserve the joint and enable foot stability.54,55 Patients are also treated with soft‐tissue and joint‐stabilising techniques as discussed for patients with CMT, but this surgery is often difficult and not without complications related to the severe sensory neuropathy and ulcers.56
Table 4 Hereditary sensory and autonomic neuropathies.
| Clinical type | Inheritance | Locus/gene |
|---|---|---|
| HSAN I/HSN1 | AD | 9q22.1–22.3/SPTLC1 |
| CMT2B | AD | 3q13–q22/RAB7 |
| HSAN I | AD | Unknown |
| HSAN IB (with cough and reflux) | AD | 3p22–24 |
| HSAN II | AR | 12p13.33/HSN2 |
| HSAN II | AR | Unknown |
| HSAN III (Riley–Day syndrome) | AR | 9q31–q33/IKAP |
| HSAN IV (CIPA) | AR | 1q21–q22/TRKA |
| HSAN V | AR | 1q21–q22/TRKA |
| HSAN V (Swedish) | AR | 1p11.2–p13.2/NGFB |
| HSAN V | AR | Unknown |
AD, autosomal dominant; AR, autosomal recessive; CIPA, congenital insensitivity to pain with anhidrosis; HSAN, hereditary sensory and autonomic neuropathy; HSN2, HSN2 gene; IKAP, IκB kinase complex‐associated protein; NGFB, nerve growth factor β‐gene; SPLTC1, serine palmitoyltransferase, long‐chain base subunit 1; TRKA, tyrosine kinase A receptor.
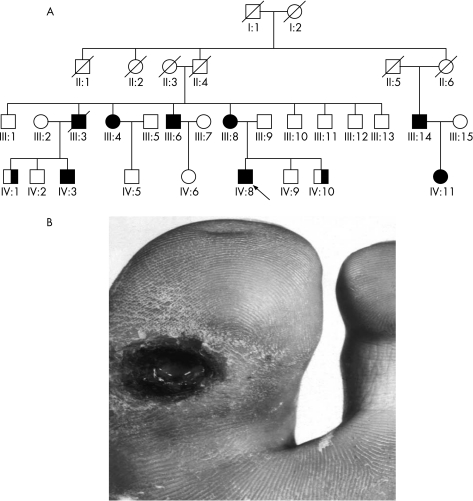
Figure 6 Typical hereditary sensory and autonomic neuropathy (HSAN) type I family tree (A). Many individuals had ulcers and amputations as shown by the big toe ulcer of patient III‐3 (B). This family had the British (C133W) mutation in the serine palmitoyltransferase long‐chain base subunit‐1 (SPTLC1) gene.
Hereditary spastic paraplegia
This disorder is frequently seen in the neurology clinic and characterised by walking problems (unsteadiness with toe walking and wearing out the front of the shoes), stiff legs, and occasionally limb weakness and sensory problems. The inheritance is usually autosomal dominant but recessive and X‐linked forms exist; patients usually have a normal lifespan. Many of the problems can be treated by home stretching, physiotherapy and drugs to treat the spasticity. In severe cases, surgery for contractures in the lower limbs, usually tenotomies, are required.
Poliomyelitis
Poliomyelitis is caused by a viral infection of the anterior horn cells of the spinal cord, which may lead to permanent paralysis of isolated groups of muscles and death. With vaccination, the acute illness is not seen in the Western world, but “old polio” is not uncommon.57 Polio tends to deteriorate later in life and some patients can develop post‐polio syndrome; the cause of this is unknown, but there are several hypothesis including neural fatigue (age‐related vulnerability of motor neurones due to previous polio infection), mitochondrial disruption and reticular activating system damage. The theory that post‐polio syndrome is due to reactivated polio has been discredited, as laboratory studies have been unable to identify the active polio virus in the body.58
After the acute stages of the illness, treatment is mainly physiotherapy and orthotic management of fixed deformities59; during the later stages, orthopaedic treatment is often required. The problems can be split into four types9:
Isolated muscle weakness without deformity: If quadriceps is weak, then walking may be helped with orthoses; elsewhere, isolated weakness may be treated by tendon transfer.
Residual deformity: Unbalanced paralysis may be corrected by orthotic management or tendon transfer, but fixed deformity requires operative stabilisation and sometimes arthrodesis.
Flail joint: Balanced paralysis may need no treatment, but unstable joints will need stabilisation by permanent orthoses or arthrodesis.
Shortening: Leg length may be compensated by a built‐up shoe. Large discrepancies may be more amenable to operative lengthening of the femur.
Patients with old polio should be followed up on a regular basis to identify deterioration where further rehabilitation or orthotic intervention is required.
Cerebral palsy and stroke
With better management, patients with cerebral palsy are living longer and are increasingly being seen by adult neurologists. The term cerebral palsy includes a group of disorders that result from non‐progressive brain damage during early development. The incidence is 1 in 1000 live births. Children usually have delayed developmental milestones and later present with a number of problems, including ataxia, dystonia, athetosis, weakness and spasticity.9,60
Orthotic aims in cerebral palsy and stroke differ dramatically according to the time from onset. In early stroke, orthoses may be used to challenge stability, optimise alignment and be used alongside physiotherapy to promote best recovery; in the chronic stages after stroke, they are more likely to be used to provide stability to maximise function.
In upper motor neurone syndromes when the peripheral nerve is intact, it may be appropriate to use functional electrical stimulation to optimise movement and function.61
A multidisciplinary lifelong treatment approach is needed, often with access to a specialist centre. Intensive home stretching and physiotherapy are required, often with muscle relaxants and phenol treatment in some patients.3,62,63,64 Surgical treatment is frequently needed. The indications for surgery are:
Deterioration or uncontrollable spastic posture
Fixed deformity that interferes with function
Secondary complications such as bony deformities, dislocation of the hip and joint instability.
Provided the deformity is controlled by other measures, there is no urgency about operations. In some cases, it may be better to delay until patients stabilise and then correct them in 1–2 sittings. The timing of procedures, as with other neuromuscular conditions, should be discussed in a patient‐centred multidisciplinary team meeting.
Surgical options are limited and mainly consist of releasing tight muscles or lengthening tendons, augmenting weak muscles by tendon transfers and correcting of fixed deformities by osteotomy, fusing the joint or by arthroplasty.65,66,67,68,69,70,71,72,73,74 Table 5 gives a list of regional operations.
Table 5 Treatment of principal limb deformities in cerebral palsy and stroke.
| Deformity | Orthotic intervention | Surgery | |
|---|---|---|---|
| Foot | Flaccid equinus | Spring‐loaded dorsiflexion | Lengthen tendo Achillis |
| Foot | Spastic equinovarus | Limiting orthosis to eversion and dorsiflexion | Lengthen tendo Achillis and transfer lateral half of tibialis anterior to cuboid |
| Knee | Flexion | Knee–ankle–foot orthosis | Hamstring release |
| Hip | Adduction | Seating adaptation | Obturator neurectomy or adductor muscle release |
| Shoulder | Adduction | Positioning pillow | Subscapularis release |
| Elbow | Flexion | Night orthosis | Release elbow flexors |
| Wrist | Flexion | Wrist orthosis | Lengthen or release wrist flexors |
| Fingers | Flexion | Night orthosis | Lengthen or release flexors |
After a stroke, there may be persistent spastic paresis and a disturbance in sensation. In the early stage, physiotherapy and splinting are important in preventing fixed contractures. Once maximal recovery has been achieved, usually by 9 months, residual deformity or joint instability may need surgical correction or permanent orthotic management.75
Unless surgery deals with the underlying muscle imbalance that induced the deformity, maintenance by orthoses or a stretching regimen will probably be necessary to prevent recurrence.
Conclusions
We have discussed the diagnostic approaches, principles of rehabilitation and the surgical techniques used in the neurological conditions commonly referred to the orthopaedic surgeon. The diagnosis of many of these disorders is rapidly changing with the latest genetic techniques, but the natural history and most surgical procedures have been well established over many years.
Until recently, no medical treatment was possible for most of the conditions that we have discussed, but recent animal in vitro studies have shown promising developments that may benefit patients. This includes an important study using high‐dose vitamin C in a mouse model of CMT1A, showing definite clinical and pathological improvement.76 Several studies have assessed the possibility of treating muscular dystrophy and other myopathies. Results of studies analysing allogeneic dystrophin‐positive myoblasts and genetically modified bone marrow‐derived mesenchymal cells that may regenerate patients' muscle tissue have been encouraging.77 There has also been work looking into the regeneration of bone, tendons and articular tissue using adult mesenchymal or embryonic stem cells; much of this work has been in vitro or in animals, but has shown promising results.78 Medical treatment to possibly regenerate or halt nerve and muscle degeneration in combination with rehabilitation and surgical procedures will hopefully revolutionise the way patients are treated and improve their quality of life.
Acknowledgements
We thank The Wellcome Trust and The Medical Research Council (MRC) for their support. We also thank the patients and families for their assistance with this work.
Abbreviations
BMD - Becker's muscular dystrophy
CMT - Charcot–Marie–Tooth disease
DMD - Duchenne's muscular dystrophy
Footnotes
Competing interests: None declared.
References
- 1.Ward C D, McIntosh S. The rehabilitation process: a neurological perspective. In: Greenwood RJ, Barnes MP, McMillan TM, Ward CD, eds. Handbook of neurological rehabilitation. 2nd edn. Hove: Psychology Press, 2003725
- 2.Reilly M M, Hanna M G. Genetic neuromuscular disease. J Neurol Neurosurg Psychiatry 200273(Suppl 2)II12–II21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood R L. The rehabilitation team. In: Greenwood RJ, Barnes MP, McMillan TM, Ward CD, eds. Handbook of neurological rehabilitation. 2nd edn. Hove: Psychology Press, 2003725
- 4.Solomon L, Warwick D, Nayagam S. Orthopaedic diagnosis. Neuromuscular disorders and peripheral nerve injuries, 8th edn. London, UK: Arnold, 2001
- 5.Greenwood R J, Barnes M P, McMillan T M, Ward C D. eds. Handbook of neurological rehabilitation. 2nd edn. Hove: Psychology Press, 2003
- 6.Edwards S, Mawson S, Greenwood R J. Physical therapies. In: Greenwood RJ, Barnes MP, McMillan TM, Ward CD, eds. Handbook of neurological rehabilitation, 2nd edn. Hove: Psychology Press, 2003725
- 7.Fyfe N C M, McClemont E J W, Panton L E.et al Assistive technology: mobility aids, environmental control systems, and communication aids. In: Greenwood RJ, Barnes MP, McMillan TM, Ward CD, eds. Handbook of neurological rehabilitation, 2nd edn. Hove: Psychology Press, 2003725
- 8.Birch J G. Orthopedic management of neuromuscular disorders in children. Semin Pediatr Neurol 1998578–91. [DOI] [PubMed] [Google Scholar]
- 9.Warner W J.Neuromuscular disorders, cerebral palsy and paralytic disorders. 10th edn. Philadelphia, PA: Mosby, 2003
- 10.Dubowitz V.Muscle disorders in childhood. 2nd edn. London: WB Saunders, 1995
- 11.Karparti G, Hilton‐Jones D, Griggs R C.Disorders of the voluntary muscle. Cambridge: Cambridge University Press, 2001
- 12.Louis D, Hensinger R, Fraser B.et al Surgical management of the severely handicapped individual. J Pediatr Orthop 1989915–18. [DOI] [PubMed] [Google Scholar]
- 13.Brown R H, Phil D. Dystrophy‐associated proteins and the muscular dystrophies. Annu Rev Med 199748457. [DOI] [PubMed] [Google Scholar]
- 14.Davies N P, Cochrane G, Hanna M. Muscle disorders. In: Greenwood RJ, Barnes MP, McMillan TM, Ward CD, eds. Handbook of neurological rehabilitation. 2nd edn. Hove: Psychology Press, 2003725
- 15.Bushby K, Muntoni F, Bourke J P. 107th ENMC International workshop: The management of cardiac involvement in muscular dystrophy and myotonic dystrophy, 7th–9th June 2002, Naarden, The Netherlands. Neuromusc Disord 200313166–172. [DOI] [PubMed] [Google Scholar]
- 16.Merlini L, Forst J. Surgical management of muscular dystrophy. In: Emery AEH, ed. The muscular dystrophies. Oxford: Oxford University Press, 2001284–296.
- 17.Siegel I M.Diagnosis, management and orthopedic treatment of muscular dystrophy. St Louis, MO: Mosby, 1981 [PubMed]
- 18.Fowler W M., Jr Rehabilitation management of muscular dystrophy and related disorders. II. Arch Phys Med Rehabil 198263322. [PubMed] [Google Scholar]
- 19.Rideau Y, Duport G, Delaubier A.et al Early treatment to preserve quality of locomotion for children with Duchenne muscular dystrophy. Semin Neurol 1995159–17. [DOI] [PubMed] [Google Scholar]
- 20.Granata C, Giannini S, Rubbini L.et al Orthopedic surgery to prolong walking in Duchenne muscular dystrophy. Chir Organi Mov 198873237–248. [PubMed] [Google Scholar]
- 21.Scheuerbrandt G. First meeting of the Duchenne Parent Project in Europe: treatment of Duchenne muscular dystrophy, 7–8 November 1997, Rotterdam, The Netherlands. Neuromusc Disord 19988213–219. [DOI] [PubMed] [Google Scholar]
- 22.Yazici M, Asher M A, Hardacker J W. The safety and efficacy of Isola‐Galveston instrumentation and arthrodesis in the treatment of neuromuscular spinal deformities. J Bone Joint Surg Am 200082524–543. [DOI] [PubMed] [Google Scholar]
- 23.Giannini S, Ceccarelli F, Faldini C.et al Surgical treatment of neck hyperextension in myopathies. Clin Orthop Relat Res 2005434151–156. [DOI] [PubMed] [Google Scholar]
- 24.Reilly M M. Classification of the hereditary motor and sensory neuropathies. Curr Opin Neurol 200013561–564. [DOI] [PubMed] [Google Scholar]
- 25.Reilly M, Greenwood R J. Disorders of the peripheral nerves. In: Greenwood RJ, Barnes MP, McMillan TM, Ward CD, eds. Handbook of neurological rehabilitation. 2nd edn. Hove: Psychology Press, 2003725
- 26.Skre H. Genetic and clinical aspects of Charcot‐Marie‐Tooth's disease. Proceedings of the Third International Congress on Muscle Diseases. Excerpta Medica International Congress Series. Number 334. Amsterdam, Excepta Medica 1974 [DOI] [PubMed]
- 27.Schwend R M, Drennan J C. Cavus foot deformity in children. J Am Acad Orthop Surg 200311201–211. [DOI] [PubMed] [Google Scholar]
- 28.Coleman S S.Complex foot deformities in children. Philadelphia, PA: Lea & Febiger, 1983
- 29.Guyton G P, Mann R A. The pathogenesis and surgical management of foot deformity in Charcot‐Marie‐Tooth disease. Foot Ankle Clin 20005317–326. [PubMed] [Google Scholar]
- 30.Olney B. Treatment of the cavus foot. Deformity in the pediatric patient with Charcot‐Marie‐Tooth. Foot Ankle Clin 20005305–315. [PubMed] [Google Scholar]
- 31.Fuller J E, DeLuca P A. Acetabular dysplasia and Charcot‐Marie‐Tooth disease in a family. A report of four cases. J Bone Joint Surg Am 1995771087–1091. [DOI] [PubMed] [Google Scholar]
- 32.Ghanem I, Zeller R, Seringe R. The foot in hereditary motor and sensory neuropathies in children. Rev Chir Orthop Reparatrice Appar Mot 199682152–160. [PubMed] [Google Scholar]
- 33.Oganesyan O V, Istomina I S, Kuzmin V I. Treatment of equinocavovarus deformity in adults with the use of a hinged distraction++ apparatus. J Bone Joint Surg Am 199678546–556. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg R S, Parker S D. Anesthetic management for the child with Charcot‐Marie‐Tooth disease. Anesth Analg 199274305–307. [DOI] [PubMed] [Google Scholar]
- 35.Mann D C, Hsu J D. Triple arthrodesis in the treatment of fixed cavovarus deformity in adolescent patients with Charcot‐Marie‐Tooth disease. Foot Ankle 1992131–6. [DOI] [PubMed] [Google Scholar]
- 36.Andruson M V, Goloborod'ko S A, Al'shanova L S. Possibilities of orthopedic treatment of hand deformities in Charcot‐Marie‐Tooth neural amyotrophy. Ortop Travmatol Protez 1988147–48. [PubMed] [Google Scholar]
- 37.Gould N. Surgery in advanced Charcot‐Marie‐Tooth disease. Foot Ankle 19844267–273. [DOI] [PubMed] [Google Scholar]
- 38.Cavuoto J W. Foot surgery in Charcot‐Marie‐Tooth disease. J Foot Surg 198019130–134. [PubMed] [Google Scholar]
- 39.Michelinakis E, Vourexakis H. Tendon transfer for intrinsic‐muscle paralysis of the thumb in Charcot‐Marie‐Tooth neuropathy. Hand 198113276–278. [DOI] [PubMed] [Google Scholar]
- 40.Riley W B, Jr, Mann R J, Burkhalter W E. Extensor pollicis longus opponensplasty. J Hand Surg [Am] 19805217–220. [DOI] [PubMed] [Google Scholar]
- 41.Miller G M, Hsu J D, Hoffer M M.et al Posterior tibial tendon transfer: a review of the literature and analysis of 74 procedures. J Pediatr Orthop 19822363–370. [PubMed] [Google Scholar]
- 42.Wetmore R S, Drennan J C. Long‐term results of triple arthrodesis in Charcot‐Marie‐Tooth disease. J Bone Joint Surg Am 198971417–422. [PubMed] [Google Scholar]
- 43.Wukich D K, Bowen J R. A long‐term study of triple arthrodesis for correction of pes cavovarus in Charcot‐Marie‐Tooth disease. J Pediatr Orthop 19899433–437. [PubMed] [Google Scholar]
- 44.Hensinger R, MacEwen G D. Spinal deformity associated with hereditable neurological conditions: spinal muscular atrophy, Friedreich's ataxia, familial dysautonomia and Charcot Marie Tooth disease. J Bone Joint Surg 197858A13. [PubMed] [Google Scholar]
- 45.Delatycki M B, Holian A, Corben L.et al Surgery for equinovarus deformity in Friedreich's ataxia improves mobility and independence. Clin Orthop Relat Res 2005430138–141. [DOI] [PubMed] [Google Scholar]
- 46.Shapiro F, Bresnan M J. Current concepts review: orthopaedic management of childhood neuromuscular disease. II. Peripheral neuropathies, Friedreich's ataxia and arthrogryposis multiplex congenita. J Bone Joint Surg 198264A949. [PubMed] [Google Scholar]
- 47.Schmidinger S, Eiber J, Schols L.et al Cardiomyopathy in patients with Friedreich's ataxia—appearance and diagnostic value. J Clin Basic Cardiol 20003167–171. [Google Scholar]
- 48.Rustin P, von Kleist‐Retsow J ‐ C, Chantrel‐Groussard K.et al Effect of idebenone on cardiomyopathy in Friedreich's ataxia: a preliminary study. Lancet 1999354477–479. [DOI] [PubMed] [Google Scholar]
- 49.Shapiro F, Bresnan M J. Current concepts review: orthopaedic management of childhood neuromuscular disease. I. Spinal muscular atrophy. J Bone Joint Surg 198264A785. [PubMed] [Google Scholar]
- 50.Granata C. Spinal muscular atrophy: natural history and orthopaedic treatment of scoliosis. Spine 198914760. [DOI] [PubMed] [Google Scholar]
- 51.Houlden H, Blake J, Reilly M M. Hereditary sensory neuropathies. Curr Opin Neurol 200417569–577. [DOI] [PubMed] [Google Scholar]
- 52.Houlden H, King R, Blake J.et al Clinical, pathological and genetic characterization of hereditary sensory and autonomic neuropathy type 1 (HSAN I). Brain 2006129(Pt 2)411–425. [DOI] [PubMed] [Google Scholar]
- 53.Laplaza F J, Turajane T, Axelrod F B.et al Nonspinal orthopaedic problems in familial dysautonomia (Riley‐Day syndrome). J Pediatr Orthop 200121229–232. [PubMed] [Google Scholar]
- 54.Warren A G. The surgical conservation of the neuropathic foot. Ann R Coll Surg Engl 198971236–242. [PMC free article] [PubMed] [Google Scholar]
- 55.Uehara K, Tsuchiya H, Kabata T.et al Ankle arthrodesis and tibial lengthening for congenital sensory neuropathy with anhidrosis. J Orthop Sci 20016430–434. [DOI] [PubMed] [Google Scholar]
- 56.Minde J, Toolanen G, Andersson T.et al Familial insensitivity to pain (HSAN V) and a mutation in the NGFB gene. A neurophysiological and pathological study. Muscle Nerve 20046752–760. [DOI] [PubMed] [Google Scholar]
- 57.Dalakas M C. The post‐polio syndrome as an evolved clinical entity. Definition and clinical description. Ann N Y Acad Sci 199575368–80. [DOI] [PubMed] [Google Scholar]
- 58.Bruno R L.The polio paradox: uncovering the hidden history of polio to understand and treat ‘post‐polio syndrome' and chronic fatigue. New York: Warner Books, 2002
- 59.Wetz H H, Exner G U. Orthoses in patients with poliomyelitis. Ther Umsch 199552483–486. [PubMed] [Google Scholar]
- 60.Brunner R, Gebhard F. Neurogenic spinal deformities. I. Conservative and surgical treatment of spinal deformities. Orthopade 20023151–57. [DOI] [PubMed] [Google Scholar]
- 61.Johnston T E, Finson R L, McCarthy J J.et al Use of functional electrical stimulation to augment traditional orthopaedic surgery in children with cerebral palsy. J Pediatr Orthop 200424283–291. [DOI] [PubMed] [Google Scholar]
- 62.Yadav S L, Singh U, Dureja G P.et al Phenol block in the management of spastic cerebral palsy. Indian J Pediatr 199461249–255. [DOI] [PubMed] [Google Scholar]
- 63.Russman B S. Cerebral palsy. Curr Treat Options Neurol 2000297–108. [DOI] [PubMed] [Google Scholar]
- 64.Wong A M, Pei Y C, Lui T N.et al Comparison between botulinum toxin type A injection and selective posterior rhizotomy in improving gait performance in children with cerebral palsy. J Neurosurg 2005102(Suppl)385–389. [DOI] [PubMed] [Google Scholar]
- 65.Marty G R, Dias L S, Gaebler‐Spira D. Selective posterior rhizotomy and soft‐tissue procedures for the treatment of cerebral diplegia. J Bone Joint Surg Am 199577713–718. [DOI] [PubMed] [Google Scholar]
- 66.Green W T., Sr Fascia grafts. Transplant Proc 19768(Suppl 1)113–118. [PubMed] [Google Scholar]
- 67.Green N E, Griffin P P, Shiavi R. Split posterior tibial‐tendon transfer in spastic cerebral palsy. J Bone Joint Surg Am 198365748–754. [PubMed] [Google Scholar]
- 68.Green D P, Parkes JC I I, Stinchfield F E. Arthrodesis of the knee. A follow‐up study. J Bone Joint Surg Am 1967491065–1078. [PubMed] [Google Scholar]
- 69.Green W T. Orthopedic management of poliomyelitis. Pediatr Clin North Am 1953135–41. [PubMed] [Google Scholar]
- 70.Kling T F, Jr, Kaufer H, Hensinger R N. Split posterior tibial‐tendon transfers in children with cerebral spastic paralysis and equinovarus deformity. J Bone Joint Surg Am 198567186–194. [PubMed] [Google Scholar]
- 71.Gage J R. Surgical treatment of knee dysfunction in cerebral palsy. Clin Orthop Relat Res 199025345–54. [PubMed] [Google Scholar]
- 72.Skoff H, Woodbury D F. Management of the upper extremity in cerebral palsy. J Bone Joint Surg Am 198567500–503. [PubMed] [Google Scholar]
- 73.Woo R. Spasticity: orthopedic perspective. J Child Neurol 20011647–53. [DOI] [PubMed] [Google Scholar]
- 74.Yalcin S, Kocaoglu B, Berker N.et al Surgical management of orthopedic problems in adult patients with cerebral palsy. Acta Orthop Traumatol Turc 200539231–236. [PubMed] [Google Scholar]
- 75.Wade D T. Stroke rehabilitation: the evidence. In: Greenwood RJ, Barnes MP, McMillan TM, Ward CD, eds. Handbook of neurological rehabilitation, 2nd edn. Hove: Psychology Press, 2003725
- 76.Passage E, Norreel J C, Noack‐Fraissignes P.et al Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot‐Marie‐Tooth disease. Nat Med 200410396–401. [DOI] [PubMed] [Google Scholar]
- 77.Goncalves M A, de Vries A A, Holkers M.et al Human mesenchymal stem cells ectopically expressing full‐length dystrophin can complement Duchenne muscular dystrophy myotubes by cell fusion. Hum Mol Genet 200615213–221. [DOI] [PubMed] [Google Scholar]
- 78.Oakes B W. Orthopaedic tissue engineering: from laboratory to the clinic. Med J Aust 2004180(Suppl)35–38. [DOI] [PubMed] [Google Scholar]