Abstract
Background
Dementia is common in Parkinson's disease, but the underlying brain pathology is not yet fully understood.
Aim
To examine the changes in the brain of patients with Parkinson's disease with mild cognitive impairment (MCI) and dementia, using structural magnetic resonance imaging.
Methods
Using voxel‐based morphometry, the grey matter atrophy on brain images of patients with Parkinson's disease and dementia (PDD; n = 16) and Parkinson's disease without dementia (PDND; n = 20), and healthy elderly subjects (n = 20) was studied. In the PDND group, 12 subjects had normal cognitive status and 8 had MCI. Standardised rating scales for motor, cognitive and psychiatric symptoms were used.
Results
Widespread areas of cortical atrophy were found in patients with PDD compared with normal controls (in both temporal and frontal lobes and in the left parietal lobe). Grey matter reductions were found in frontal, parietal, limbic and temporal lobes in patients with PDD compared with those with PDND. In patients with PDND with MCI, areas of reduced grey matter in the left frontal and both temporal lobes were found.
Conclusion
These findings show that dementia in Parkinson's disease is associated with structural neocortical changes in the brain, and that cognitive impairment in patients with PDND may be associated with structural changes in the brain. Further studies with larger groups of patients are needed to confirm these findings.
Cognitive impairment and neuropsychiatric symptoms are common in patients with Parkinson's disease, and most patients with Parkinson's disease who survive >10 years after the onset of Parkinson's disease will eventually develop dementia (PDD).1 The dementia profile of PDD is characterised by executive, visuospatial and attentional impairment, suggesting that frontostriatal changes are the main causes of dementia in patients with Parkinson's disease.2,3 In addition, a substantial proportion of patients with Parkinson's disease have mild cognitive impairment (MCI) without fulfilling dementia criteria, even early in the course of disease.4,5 Preliminary evidence suggests that these mild cognitive changes represent the early stages of dementia.6,7
The few existing studies of Parkinson's disease using structural magnetic resonance imaging (MRI) techniques have not consistently shown a specific pattern of atrophy in patients with PDD. In a study measuring hippocampal volumes,8 patients with Parkinson's disease with and without cognitive impairment had more hippocampal atrophy than healthy age‐matched controls. Another volumetric study found reduction in volume of both hippocampus and amygdala in patients with PDD and patients with Parkinson's disease without dementia (PDND) compared with normal controls.9 These findings were confirmed in a recent study using visual rating of the medial temporal lobes. There were no significant differences in hippocampal atrophy in patients with PDD compared with those with PDND.10 Patients belonging to both Parkinson's disease groups, however, showed more hippocampal atrophy than the control group.
In a study using voxel‐based morphometry (VBM), neocortical atrophy has also been reported in patients with PDD. VBM is an automated, unbiased method for voxel‐wise comparison of anatomical data from high‐resolution MRI. The method is fairly new, introduced in 2000,11 and compares the density of grey or white matter between groups of individuals. In patients with PDD, reduced grey matter volume in temporal and occipital lobes, right frontal and left parietal lobes were found, together with subcortical areas.12 Similar but less widespread atrophy was reported in a recent study, which also found hippocampal atrophy in patients with PDD.13
Changes in MRI have also been reported in patients with PDND using VBM. Reduced volumes in the right frontal lobe,12 temporal lobe and hippocampus,13 and limbic and parahippocampal atrophy have been found.14
Two longitudinal studies have recently been published on the rates of atrophy in patients with Parkinson's disease versus controls. Whole‐brain atrophy rates were significantly increased in patients with PDD compared with those with PDND and controls.15 No increase in atrophy in patients with PDND compared with normal controls was found. Another longitudinal study using VBM found progressive decreases in the grey matter volume in limbic, paralimbic and temporo‐occipital regions in patients with PDND, and, neocortical grey matter loss was found in patients with PDD.16
No MRI studies of patients with Parkinson's disease and MCI have, to our knowledge, yet been reported. Thus, whether structural changes exist in patients with Parkinson's disease and MCI, and whether the previously reported cortical and hippocampal atrophy is present in all patients with PDND or only in a subgroup with MCI is not clear.
To explore these issues, we investigated structural brain MRIs of patients with Parkinson's disease with and without MCI and dementia and healthy elderly volunteers using VBM.
We hypothesised that patients with PDD would have more pronounced cortical atrophy than controls and patients with PDND, and that those with Parkinson's disease with MCI would show more atrophy than those with Parkinson's disease with intact cognition. On the basis of the cognitive profile of PDD and previous MRI studies, we further hypothesised that these changes in the brain would involve frontal, limbic and temporal lobes, including the medial temporal cortex.
Patients and methods
Case‐finding and diagnostic procedures
The patients were recruited from an ongoing epidemiological study (sample 1).17 In addition, consecutive patients referred to outpatient clinics of the Department of Neurology or the Department of Geriatric Psychiatry, Stavanger University Hospital, Stavanger, Norway, were included (sample 2). A diagnosis of Parkinson's disease was made by a neurologist, according to explicit criteria.18 The minimum requirement for a diagnosis of Parkinson's disease was at least two of the cardinal signs (akinesia, rigidity, resting tremor or postural abnormalities) and a moderate response to a dopaminergic agent. Staging of Parkinson's disease was carried out according to the Hoehn and Yahr scale.19 Sample 1 has been followed up prospectively with a 4‐year interval in 1993, 1997 and 2001 and then assessed annually. The autopsy diagnosis in the first 22 patients, 2 of whom participated in the current MRI study, was consistent with a diagnosis of Lewy‐body Parkinson's disease.20
Diagnosis of dementia
The diagnosis of dementia was based on a semistructured interview with the patient and a care giver following the Diagnostic and Statistical Manual of Mental Disorders‐III‐R criteria for dementia21 (sample 1) or Diagnostic and Statistical Manual of Mental Disorders IV (sample 2).22 All patients completed the Mini‐Mental State Examination (MMSE).23 In addition, sample 1 completed the Dementia Rating Scale.24 Patients with an MMSE score ⩾16 completed a neuropsychological battery consisting of (1) the multiple‐choice version of the Benton Visual Retention Test,25 the Judgement of Line Orientation Test26 and the Stroop Word Test.27 These neuropsychological tests were selected to identify cognitive deficits typically occurring in Parkinson's disease,28 and were as much as possible independent of motor abilities. The tests were administered by a neuropsychologist, and scored according to conventional procedures outlined in the test manuals. A detailed description of the test battery is made elsewhere.5 Sample 2 completed the Cambridge Cognitive Examination, the cognitive section of the Cambridge Mental Disorders of the Elderly Examination.29 To qualify for a diagnosis of dementia, the interview and the cognitive rating scales had to be compatible with a diagnosis of dementia. The final diagnosis was made by one of the authors (DA) on the basis of all available information except the MRI scan. Further details of the clinical assessment of sample 1 can be found in Tandberg et al30 and Aarsland et al.31 Psychiatric symptoms were assessed using the Neuropsychiatric Inventory32 in all patients with Parkinson's disease with cognitive impairment to detect cognitive impairment secondary to psychiatric syndromes such as depression.
Mild cognitive impairment
MCI was defined according to the criteria proposed by Petersen et al33: impaired performance (ie, ⩾1½ standard deviation (SD) below the mean of a control group matched with the PDND group for age, sex and education) on one, two or all three neuropsychological tests. In addition, information regarding memory problems or other subjective cognitive deficits was gathered by means of the care giver‐based dementia interview and the mentation item from the mental subscale of Unified Parkinson Disease Rating Scale.34 MCI was diagnosed if cognitive impairment on testing and from the interview was not severe enough to affect activities of daily living, and thus the criteria for dementia were not met. For a more detailed description of the MCI diagnosis, see Janvin et al.7
Control group
Healthy elderly controls were recruited from the local Parkinson interest group, elderly people from local clubs for retired people and from relatives of patients with Parkinson's disease or other neuropsychiatric disorders. The controls had no active neurological or psychiatric disorder. They had no cognitive deficits, and were not taking drugs that could affect their cognition. A minimum MMSE score of 28 was required. Written consent was obtained from all patients and controls, and the study was approved by Regional Committee for Medical Research Ethics, University of Bergen, Bergen, Norway.
Exclusion criteria
Patients with other known brain disorders apart from Parkinson's disease, Parkinsonism due to antipsychotic or other drugs, or a history of schizophrenia or bipolar disorder were not included. To avoid inclusion of patients with dementia with Lewy bodies, patients with clinical signs of cognitive impairment during the first year after onset of Parkinson's disease were excluded according to the International Consensus Criteria.35 We checked the standard sequences of the MRI scans (T1, dual fast field echo (FFE) and T2 flair) before the inclusion of a patient or control, and those who had structural abnormalities in the brain affecting the grey matter were excluded from VBM analysis. Patients with marked tremor, which interferes with the imaging session and produces movement artefacts, were also excluded.
Magnetic resonance imaging
The subjects were scanned at the Department of Radiology, Stavanger University Hospital, in the period from December 2001 to June 2005, in a 1.5‐T Phillips Gyroscan NT intra release 8.1 (Philips Medical Systems, Best, The Netherlands). The software of the machine was upgraded in the autumn of 2003, to Release 10. This has not affected the quality of the images, which has been stable throughout the study. We performed a structural MRI series with a T1‐weighted three‐dimensional fast, spoiled gradient recalled echo (TR 12.4 ms, TE 4.2 ms, TI 650 ms, matrix 256×192, slice thickness 1.6 mm).
Image analysis
Standard sequences (T1, dual FFE, T2 flair) were examined to visualise focal lesions of grey matter that might lead to exclusion of patients from the study. These sequences were not used for statistical image analysis in this study, but results on visual rating of white matter hyperintensities can be found in Beyer et al.36
VBM preprocessing
The optimised VBM protocol of Good et al37 was applied for the preprocessing of the images, including creating a study‐specific T1 template image (based on all the patients and controls in our study) and a study‐specific grey matter template/prior probability map. The preprocessing steps have already been described in detail by others.12,37 The first preprocessing step includes the creation of the customised templates. T1 images of each patient were normalised to the T1 template of statistical parametric mapping (SPM)2 using an affine only cut‐off. After normalising, images were averaged and then smoothed with an 8 mm kernel, creating the T1 template. Normalising the original images to the customised T1 template using a 25 mm cut‐off created the customised grey matter template. The normalised images were then segmented and smoothed with an 8 mm kernel. The smoothed grey matter images were then averaged, creating the study‐specific grey matter template. These templates were used in the optimised VBM protocol in the following manner: the original images were segmented and the grey matter images were normalised to the customised grey matter template. The resulting normalisation parameters were used to normalise the original T1 images before the final segmentation. Segmented images were then smoothed with a 12 mm kernel. The resulting smoothed images were used in the statistical analysis.
Statistical analyses
The smoothed images from the preprocessing steps were analysed using SPM2 (Wellcome Department of Cognitive Neurology, London, UK (http://www.fil.ion.ucl.ac.uk/spm)). Smoothed images that are used to show regional changes in grey matter, above that occurring globally, are referred to as unmodulated images.
The following VBM analyses of grey matter were performed: for unmodulated images, we statistically assessed differences in grey matter between groups using one‐way analysis of variance (ANOVA). We also performed analyses where age, sex and duration of Parkinson's disease were included as covariates in analysis of covariance (ANCOVA). For all analyses, the whole brain was analysed. As we had a prior hypothesis about the localisation of change, significance levels for t statistics were set at p<0.001 uncorrected. All the results are presented at the voxel level.38 In addition, we performed a small volume correction (SVC) using the WFU PickAtlas software V.2,39,40 and corrected for multiple comparisons using p family‐wise error (FWE) <0.05. The SVC reduces the number of voxels entering the statistical computation by defining a region of interest. The correction for multiple comparisons is based on the number of voxels in the region of interest.
The coordinates obtained for the peak voxels—that is, the anatomical location with maximal grey matter loss in each significant cluster—were transferred into Talairach space using Matthew Brett's mni2tal routine (http://www.mrc‐cbu.cam.ac.uk/imaging/index.html).
The anatomical locations of the peak voxels were then found using The Talairach Daemon Client.41 The results given by the Talairach Daemon were verified from the Co‐planar stereotaxic atlas of the human brain.42 Data were analysed on a personal computer using Windows XP Professional V.5.1 and Matlab 6.5.2 and SPM2 (http://www.fil.ion.ucl.ac.uk/spm).
Group statistics were analysed using SPSS for Windows V.12.0.1. Differences between groups on continuous variables with normal distribution were assessed using one‐way ANOVA with retrospective Scheffe's test to determine group differences. For the non‐parametric data a Kruskal–Wallis test was used, followed by a retrospective Mann–Whitney U test, or a χ2 test, when appropriate, using p<0.05 as significant.
Results
A total of 56 participants were included: PDD (sample 1, n = 3; sample 2, n = 13), PDND (sample 1, n = 14; sample 2, n = 6) and normal controls (n = 20). Table 1 presents the demographic and clinical characteristics. We found no significant differences in age, education, duration of illness or sex between the three groups. As expected, patients with PDD had a lower MMSE score and a higher Hoehn and Yahr stage than those with PDND. The mean duration of dementia in the PDD group was 1.7 (SD 0.8) years. In the PDND group, 12 subjects had normal cognitive status and 8 had MCI (5 were impaired on 1 test, 2 on 2 and 1 on all 3 tests; table 2). The patients with MCI had a lower mean duration of education (p = 0.013; table 2) and a non‐significant trend towards higher age (p = 0.057). As expected, patients with MCI had a lower MMSE (p = 0.007) compared with those with a normal cognitive status (table 2).
Table 1 Characteristics of participants.
| PDD | PDND | Controls | p Value | |
|---|---|---|---|---|
| Participants | 16 | 20 | 20 | NA |
| Female/male | 6/10 | 11/9 | 10/10 | 0.56 |
| Mean age | 73.5 (6.5) | 72.5 (8.5) | 73.3 (6.3) | 0.9 |
| Mean MMSE score | 19.4 (4.6) | 28.2 (2.1) | 29.6 (0.7) | <0.001* |
| Mean education (years) | 10.2 (3.6) | 11.0 (3.6) | 12.1 (4.3) | 0.31 |
| Mean H&Y stage | 3.0 (0.6) | 2.4 (0.6) | NA | 0.008† |
| Mean duration of PD (years) | 12.3 (7.5) | 12.0 (6.3) | NA | 0.38 |
H&Y stage, Hoehn & Yahr stage; MMSE, Mini Mental State Examination; NA, non‐applicable; PD, Parkinson's disease; PDD, Parkinson's disease with dementia; PDND, Parkinson's disease without dementia.
*Significantly lower mean value in PDD compared with PDND and controls.
†Higher H&Y stage in PDD compared with PDND.
Standard deviation is given in parentheses.
Table 2 Characteristics of patients with Parkinson's disease without dementia.
| MCI | No MCI | p Value | |
|---|---|---|---|
| Participants | 8 | 12 | NA |
| Female/male | 5/3 | 6/6 | 0.47* |
| Mean age (years) | 77.4 (7.4) | 69 (8) | 0.057 |
| Mean MMSE score | 25.9 (2.9) | 29.4 (0.5) | 0.007 |
| Mean education (years) | 8.4 (1.5) | 12.5 (3.6) | 0.013 |
| Mean H&Y stage | 2.6 (0.8) | 2.3 (0.4) | 0.52 |
| Mean duration of PD (years) | 10.8 (3.7) | 14.1 (7.1) | 0.52 |
H&Y stage, Hoehn and Yahr stage; MCI, mild cognitive impairment; MMSE, Mini‐Mental State Examination; NA, non‐applicable; PD, Parkinson's disease.
*Fishers exact test.
Standard deviation is given in parentheses.
Eight patients were recruited for MRI, but not included in the study. Two patients became agitated during the scanning, two patients had movement artefacts, three had clinically unrecognised structural lesions, such as a cortical infarction or a post‐traumatic lesion, and one patient could not be scanned in the correct head position. One control was excluded because of claustrophobia. Mean (SD) time between clinical testing and MRI scanning was 69 (19.8) days for sample 1 and 40 (23.1) days for sample 2.
MRI changes in patients with PDND and PDD
Patients with PDD versus controls
Patients with PDD had reductions in grey matter concentration in the limbic lobes (amygdala) and both temporal lobes, compared with the controls. On the left side, there was also reduced grey matter density in the frontal lobe, limbic lobe (cingulate and hippocampus) and brain stem red nucleus. On the right side, there was reduced grey matter density in the middle occipital gyrus (see supplemental table A at http://jnnp.bmjjournals.com/supplemental). When we included age, sex and Parkinson's disease duration as covariates in ANCOVA, the results were unchanged. We found no areas where controls had more grey matter atrophy than patients with PDD. Using an SVC and correcting for multiple comparisons, the bilateral reduction in grey matter in the middle temporal gyrus and amygdala, and also in the left brain stem red nucleus, was significant at p FWE <0.05.
Patients with PDD compared with those with PDND
In patients with PDD, there were areas of marked grey matter reduction in the frontal lobes, limbic, parietal and temporal lobes bilaterally. On the right side, there was also reduced grey matter density in the pulvinar of the thalamus (see supplementary table B at http://jnnp.bmjjournals.com/supplemental). The areas surviving SVC with correction for multiple comparisons using FWE are marked with an asterisk in the table. The results did not change when age, sex and duration of Parkinson's disease were included as covariates in ANCOVA.
There were no areas where patients with PDND had more grey matter atrophy than patients with PDD.
Patients with PDND compared with normal controls
Patients with PDND had a cluster of reduced grey matter in the right superior temporal gyrus compared with normal controls. The result was unchanged when covariates were included, but was not significant after SVC and correction for multiple comparisons. Normal controls did not have any areas of more grey matter atrophy than PDND.
Group comparison of patients with PDND with and without MCI
Patients with Parkinson's disease with MCI had reduced cortical grey matter density compared with those with cognitively intact Parkinson's disease in the left middle frontal gyrus, precentral gyrus, left superior temporal lobe and right inferior temporal lobe (fig 1, table 3). The findings were not significant after SVC and correction for multiple comparisons. When we analysed with age and sex as covariates in ANCOVA, the changes were no longer present, but when analysed with duration of Parkinson's disease as covariate the same changes in grey matter were found as in the ANOVA. Patients with Parkinson's disease without MCI did not have any areas of more grey matter atrophy than patients with Parkinson's disease with MCI.
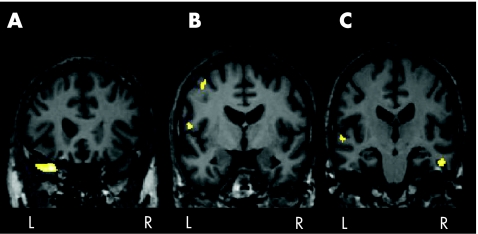
Figure 1 Areas of reduced grey matter in the analysis of unmodulated images of patients with Parkinson's disease with mild cognitive impairment (MCI) compared with those who had no cognitive impairment. The figure shows areas of regional changes in grey matter, above that occurring globally, in patients with Parkinson's disease without dementia and MCI. Significant changes are found in the (A) left superior temporal gyrus, (B) left frontal lobe (precentral gyrus) and (C) right temporal lobe (inferior temporal gyrus) and left temporal lobe (superior temporal gyrus) at p<0.001 uncorrected. L, left side, R, right side.
Table 3 Anatomical location of areas of reduced grey matter in patients with Parkinson's disease with mild cognitive impairment compared with no cognitive impairment.
| Cluster size | Voxel level | Anatomical location | x | y | z | T score |
|---|---|---|---|---|---|---|
| 420 | L | Superior temporal | −30 | 21 | −28 | 5.31 |
| 103 | L | Precentral gyrus | −58 | −5 | 11 | 4.75 |
| 75 | R | Inferior temporal | 55 | −20 | −21 | 4.49 |
| 79 | L | Superior temporal | −62 | −22 | 3 | 4.27 |
| 107 | L | Precentral gyrus | −42 | −5 | 56 | 4.08 |
| L | Middle frontal | −39 | 3 | 58 | 3.74 |
L, left; R, right.
The coordinates x, y and z refer to the anatomical location, indicating standard stereotactic space as defined by Talairach and Tournoux.42 Only clusters >200 mm3 are included. In this table, all reported voxels are p uncorrected <0.001.
Discussion
We studied grey matter changes in patients with Parkinson's disease with MCI and dementia using structural MRI and VBM. The main finding was the widespread reduced density of cortical grey matter in patients with PDD compared with controls and patients with PDND. Frontal, temporal and limbic lobes, including the medial temporal cortex, were affected in patients with PDD, in accordance with our hypothesis. They also had reduced grey matter concentration in the parietal cortices and the right thalamus when compared with patients with PDND. We found grey matter reduction in the hippocampus (right side) and amygdala (bilaterally) in the PDD group. This confirms the results from previous studies with different methods of MRI analysis.9,12
Our results are similar to but not identical with, those reported in a previous study using VBM.12 In that study, patients with PDD showed bilateral frontal, temporal and occipital grey matter volume loss relative to controls, whereas the changes between PDND and PDD groups were confined to the occipital lobes. There are several possible explanations for these differences. The patients with PDD in the two studies were comparable with regard to age and overall severity of dementia as measured with the MMSE, but the PDD group in the previous study had a markedly shorter disease duration compared with our cohort. Thus, that cohort had a later age at onset of Parkinson's disease, and an earlier and more rapid cognitive decline than our cohort. Dementia early in the course of Parkinson's disease has often been shown to represent other brain disorders,43 and preliminary data indicate differential changes in the brain underpinning dementia occurring within the first 10 years after the onset of Parkinson's disease compared with after >10 years.44 Different underlying changes in the brain related to differences in the time to develop dementia may therefore explain the MRI findings in these two studies. Another possible explanation is the higher statistical power in the previous study due to a larger sample size (n = 83) than in our study (n = 56). This hypothesis is supported by a recent report finding less widespread neocortical atrophy with an even smaller sample size (n = 42).13 The small sample size of the groups studied limits the possibility of generalising from these results.
Another potential explanation for the contrasting results among these studies may be the differential selection process of patients; about half of our sample was recruited from a community‐based study and the rest from patients referred to outpatient clinics, compared with samples based on only referrals to specialist clinics in the other studies. In line with this, the patients with Parkinson's disease in the Spanish study45 were younger than the patients in our study. There were also differences in sex distribution, although sex‐specific differences in MRI in Parkinson's disease have not been shown. Finally, information of the detailed cognitive profile in the two studies was not available. Despite similar overall dementia severity, differences in the cognitive profile—for example, relative severity of frontal, visuospatial and memory type cognitive disturbances—were observed in groups of patients with Parkinson's disease and PDD,4,46 which may be related to differences in the severity and distribution of cortical atrophy.
As motor disease severity in Parkinson's disease was greater in the PDD group than the PDND group, the differences observed in cortical atrophy may be merely associated with the different motor disease stage. When controlling for Hoehn & Yahr stage in the analysis of patients with PDND versus those with PDD, we still found the same areas of atrophy. This indicates that our findings cannot be explained entirely by the increased motor disease severity in PDD.
A novel finding was the cortical grey matter reduction in patients with PDND with MCI compared with patients with Parkinson's disease with normal cognition.
Several studies have shown that cognitive impairment, in particular executive and attentional dysfunction, is common even in patients with PDND.4,46 In contrast with a previous study,12 which reported widespread cortical changes also in patients with PDND, we found only minimal differences in patients with PDND compared with controls. However, when the patients with PDND were classified into groups with or without MCI, we found areas of grey matter atrophy in the MCI group compared with the cognitively intact group. These results were in accordance with our hypothesis.
In this study, the grey matter atrophy in Parkinson's disease with MCI was found in the ANOVA, and in the ANCOVA with Parkinson's disease duration as covariate, whereas the differences were not present when age was entered as a covariate. This could be due to the low power with 8 v 12 patients in the compared groups, and even lower degrees of freedom with a covariate in the analysis, or that atrophy is due to the age difference between groups. However, as changes were found in some of the same areas as in the PDD group, but less widespread, this may indicate that MCI in Parkinson's disease is associated with cortical atrophy and may represent early dementia. Similar results from other studies using VBM and volumetry in subjects without Parkinson's disease with MCI support our view. Atrophy in areas that are involved early in Alzheimer dementia, such as the hippocampus and temporal neocortex,47,48 and in the hippocampal region and cingulate gyri49 has been reported.
The main limitations of this study is the small sample size, particularly of patients with Parkinson's disease with MCI, the possibility of selection bias, as a proportion of the patients with Parkinson's disease were referrals to outpatient hospital clinics, and the lack of pathological confirmation of the clinical diagnosis of Parkinson's disease in most cases.
Future imaging studies and clinicopathological studies with adequate sample size are needed to further explore the relationship between specific cognitive deficits and the underlying brain correlates in Parkinson's disease, and to see whether MRI can contribute in the early diagnosis of dementia in Parkinson's disease.
Supplementary Material
Acknowledgements
We thank Borje Stangeland, PACS‐coordinator, Stavanger University Hospital, for invaluable help with image transfer, PC and software. We thank: the technicians responsible for the acquisition of MRI, Bent Erdal, Eldbjorg Finnestad, Oystein Kallevaag, Frederikke Wick, Signe Steinnes, Einar Kaarstad and Bente P Vatland; Karsten Specht, PhD, Department of Biological and Medical Psychology & National Competence Centre for Functional MRI, University of Bergen, for help with statistical analysis in VBM and interpretation of results; Emma Burton, PhD, Wolfson Research Centre, Institute for Ageing and Health, Newcastle General Hospital, UK, for help with the procedure of preprocessing images in SPM2; and Jonny Beyer for help with preparing the manuscript for submission.
Abbreviations
ANCOVA - analysis of covariance
ANOVA - analysis of variance
FWE - family‐wise error
MCI - mild cognitive impairment
MMSE - Mini‐Mental State Examination
MRI - magnetic resonance imaging
PDD - Parkinson's disease with dementia
PDND - Parkinson's disease without dementia
SPM - statistical parametric mapping
SVC - small volume correction
VBM - voxel‐based morphometry
Footnotes
Funding: This work was funded by Western Norway Regional Health Authority.
Competing interests: None.
References
- 1.Aarsland D, Andersen K, Larsen J P.et al Prevalence and characteristics of dementia in Parkinson disease: an 8‐year prospective study. Arch Neurol 200360387–392. [DOI] [PubMed] [Google Scholar]
- 2.Emre M. Dementia associated with Parkinson's disease. Lancet Neurol 20032229–237. [DOI] [PubMed] [Google Scholar]
- 3.Aarsland D, Litvan I, Salmon D.et al Performance on the dementia rating scale in Parkinson's disease with dementia and dementia with Lewy bodies: comparison with progressive supranuclear palsy and Alzheimer's disease. J Neurol Neurosurg Psychiatry 2003741215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foltynie T, Brayne C E, Robbins T W.et al The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPaIGN study. Brain 2004127550–560. [DOI] [PubMed] [Google Scholar]
- 5.Janvin C C, Aarsland D, Larsen J P. Cognitive predictors of dementia in Parkinson's disease: a community‐based, 4‐year longitudinal study. J Geriatr Psychiatry Neurol 200518149–154. [DOI] [PubMed] [Google Scholar]
- 6.Levy G, Jacobs D M, Tang M X.et al Memory and executive function impairment predict dementia in Parkinson's disease. Mov Disord 2002171221–1226. [DOI] [PubMed] [Google Scholar]
- 7.Janvin C C, Larsen J P, Aarsland D.et al Subtypes of mild cognitive impairment in Parkinson's disease: progression to dementia. Mov Disord 2006211343–1349. [DOI] [PubMed] [Google Scholar]
- 8.Camicioli R, Moore M M, Kinney A.et al Parkinson's disease is associated with hippocampal atrophy. Mov Disord 200318784–790. [DOI] [PubMed] [Google Scholar]
- 9.Junque C, Ramirez‐Ruiz B, Tolosa E.et al Amygdalar and hippocampal MRI volumetric reductions in Parkinson's disease with dementia. Mov Disord 200520540–544. [DOI] [PubMed] [Google Scholar]
- 10.Tam C W, Burton E J, McKeith I G.et al Temporal lobe atrophy on MRI in Parkinson disease with dementia: a comparison with Alzheimer disease and dementia with Lewy bodies. Neurology 200564861–865. [DOI] [PubMed] [Google Scholar]
- 11.Ashburner J, Friston K J. Voxel‐based morphometry—the methods. Neuroimage 20006(Pt 1)805–821. [DOI] [PubMed] [Google Scholar]
- 12.Burton E J, McKeith I G, Burn D J.et al Cerebral atrophy in Parkinson's disease with and without dementia: a comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain 2004127791–800. [DOI] [PubMed] [Google Scholar]
- 13.Summerfield C, Junque C, Tolosa E.et al Structural brain changes in Parkinson disease with dementia: a voxel‐based morphometry study. Arch Neurol 200562281–285. [DOI] [PubMed] [Google Scholar]
- 14.Nagano‐Saito A, Washimi Y, Arahata Y.et al Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology 200564224–229. [DOI] [PubMed] [Google Scholar]
- 15.Burton E J, McKeith I G, Burn D J.et al Brain atrophy rates in Parkinson's disease with and without dementia using serial magnetic resonance imaging. Mov Disord 2005201571–1576. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez‐Ruiz B, Marti M J, Tolosa E.et al Longitudinal evaluation of cerebral morphological changes in Parkinson's disease with and without dementia. J Neurol 20052521345–1352. [DOI] [PubMed] [Google Scholar]
- 17.Tandberg E, Larsen J P, Nessler E G.et al The epidemiology of Parkinson's disease in the county of Rogaland, Norway. Mov Disord 199510541–549. [DOI] [PubMed] [Google Scholar]
- 18.Larsen J P, Dupont E, Tandberg E. Clinical diagnosis of Parkinson's disease. Proposal of diagnostic subgroups classified at different levels of confidence. Acta Neurol Scand 199489242–251. [DOI] [PubMed] [Google Scholar]
- 19.Hoehn M M, Yahr M D. Parkinsonism: onset, progression and mortality. Neurology 196717427–442. [DOI] [PubMed] [Google Scholar]
- 20.Aarsland D, Perry R, Brown A.et al Neuropathology of dementia in Parkinson's disease: a prospective, community‐based study. Ann Neurol 200558773–776. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association Diagnostic and statistical manual of mental disorders (DSM III). Washington, DC: APA, 1987
- 22.American Psychiatric Association Diagnostic and statistical manual of mental disorders, DSM IV. 4th edn. Washington, DC: APA, 1996
- 23.Folstein M F, Folstein S E, Mc Hugh P R. “Mini‐mental State.” A practical method for grading the mental state of patients for the clinician. J Psychiatr Res 197512189–198. [DOI] [PubMed] [Google Scholar]
- 24.Mattis S.Dementia Rating Scale. New York: Grune & Stratton, 1976
- 25.Benton A L.The revised visual retention test. 4th edn. New York: Psychological Corporation, 1974
- 26.Benton A L, Varney N R, Hamsher K D. Visuospatial judgment. A clinical test. Arch Neurol 197835364–367. [DOI] [PubMed] [Google Scholar]
- 27.Golden Stroop color and word test. Chicago, IL: Stoelting, 1978
- 28.Dubois B, Pillon B. Cognitive deficits in Parkinson's disease. J Neurol 19972442–8. [DOI] [PubMed] [Google Scholar]
- 29.Roth M, Tym E, Mountjoy C Q.et al CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 1986149698–709. [DOI] [PubMed] [Google Scholar]
- 30.Tandberg E, Larsen J P, Aarsland D.et al The occurrence of depression in Parkinson's disease. A community‐based study. Arch Neurol 199653175–179. [DOI] [PubMed] [Google Scholar]
- 31.Aarsland D, Andersen K, Larsen J P.et al Risk of dementia in Parkinson's disease: a community‐based, prospective study. Neurology 200156730–736. [DOI] [PubMed] [Google Scholar]
- 32.Cummings J L, Mega M, Gray K.et al The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994442308–2314. [DOI] [PubMed] [Google Scholar]
- 33.Petersen R C, Doody R, Kurz A.et al Current concepts in mild cognitive impairment. Arch Neurol 2001581985–1992. [DOI] [PubMed] [Google Scholar]
- 34.Fahn S, Elton R L. UPDRS Program Members. Unified Parkinson's disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson's disease. Vol 2. Florham Park, NJ: Macmillan Health Care Information, 1987153–163.
- 35.McKeith I G, Galasko D, Kosaka K.et al Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996471113–1124. [DOI] [PubMed] [Google Scholar]
- 36.Beyer M K, Aarsland D, Greve O J.et al Visual rating of white matter hyperintensities in Parkinson's disease. Mov Disord 200621223–229. [DOI] [PubMed] [Google Scholar]
- 37.Good C D, Johnsrude I S, Ashburner J.et al A voxel‐based morphometric study of ageing in 465 normal adult human brains. NeuroImage 20011421–36. [DOI] [PubMed] [Google Scholar]
- 38.Friston K J, Holmes A, Poline J B.et al Detecting activations in PET and fMRI: levels of inference and power. Neuroimage 19964223–235. [DOI] [PubMed] [Google Scholar]
- 39.Maldjian J A, Laurienti P J, Kraft R A.et al An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 2003191233–1239. [DOI] [PubMed] [Google Scholar]
- 40.Maldjian J A, Laurienti P J, Burdette J H. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 200421450–455. [DOI] [PubMed] [Google Scholar]
- 41.Lancaster J L, Woldorff M G, Parsons L M.et al Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 200010120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talairach J, Tournoux P.Co‐planar stereotaxic atlas of the human brain. 3‐dimensional proportional system: an approach to cerebral imaging. Thieme Medical Publishers, New York:, 1988
- 43.Litvan I, Paulsen J S, Mega M S.et al Neuropsychiatric assessment of patients with hyperkinetic and hypokinetic movement disorders. Arch Neurol 1998551313–1319. [DOI] [PubMed] [Google Scholar]
- 44.Aarsland D, Perry E, Perry R.et al Duration of parkinsonism prior to dementia is associated with a different pattern of neuropathological and neurochemical substrates in DLB and PDD. World Parkinson Congress. Mov Disord 200621(Suppl 13)S96 [Google Scholar]
- 45.Summerfield C, Gomez‐Anson B, Tolosa E.et al Dementia in Parkinson disease: a proton magnetic resonance spectroscopy study. Arch Neurol 2002591415–1420. [DOI] [PubMed] [Google Scholar]
- 46.Janvin C C, Larsen J P, Salmon D P.et al Cognitive profiles of individual patients with Parkinson's disease and dementia: comparison with dementia with lewy bodies and Alzheimer's disease. Mov Disord 200621337–342. [DOI] [PubMed] [Google Scholar]
- 47.Pennanen C, Testa C, Laakso M P.et al A voxel based morphometry study on mild cognitive impairment. J Neurol Neurosurg Psychiatry 20057611–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pennanen C, Kivipelto M, Tuomainen S.et al Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging 200425303–310. [DOI] [PubMed] [Google Scholar]
- 49.Chetelat G, Desgranges B, De La Sayette V.et al Mapping gray matter loss with voxel‐based morphometry in mild cognitive impairment. Neuroreport 2002131939–1943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.