Abstract
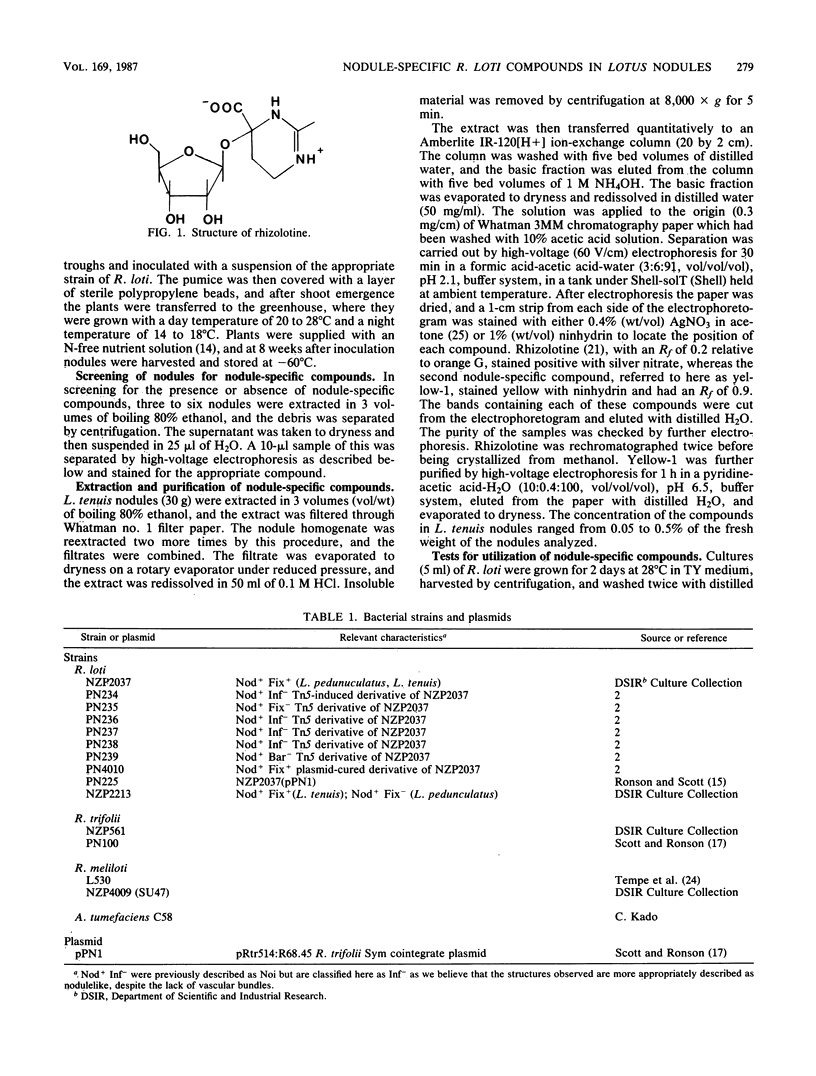
Two nodule-specific Rhizobium loti compounds were identified in Lotus tenuis and Lotus pedunculatus nodules induced by strain NZP2037. One, a silver nitrate-positive cation called rhizolotine, has been characterized as the riboside of a novel alpha-hydroxyimino acid containing a 1,4,5,6-tetrahydropyrimidine ring (G. J. Shaw, R. D. Wilson, G. A. Lane, L. D. Kennedy, D. B. Scott, and G. J. Gainsford, J. Chem. Soc. Chem. Commun., p. 180-181, 1986), and the other, yellow-1, stains yellow with ninhydrin. Both compounds were degraded by R. loti NZP2037 but not by strains of Rhizobium meliloti, Rhizobium trifolii, or Agrobacterium tumefaciens. Under the conditions tested neither compound was able to serve as a sole source of C or N for growth of R. loti NZP2037. Rhizolotine and yellow-1 were found in nodules from a range of different legumes inoculated with NZP2037, suggesting that the Rhizobium and not the host plant determines their synthesis. Neither compound was found in nodulelike structures of L. pedunculatus induced by transposon Tn5-induced noninfectious (Inf-) mutants of NZP2037 or in similar structures induced by a transconjugant of NZP2037 containing the symbiotic (Sym) cointegrate plasmid pPN1 of R. trifolii. Both compounds were also absent in the ineffective nodules induced by the bacterial-release-negative (Bar-) mutant, strain PN239. However, both compounds were present in nodules induced by the fixation-negative (Fix-) mutant PN235 and in Fix+ nodules formed by a plasmid-cured derivative of NZP2037. These results would suggest that infection and bacterial release from the infection thread are necessary for nodule (symbiotic) synthesis of these compounds.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Chua K. Y., Pankhurst C. E., Macdonald P. E., Hopcroft D. H., Jarvis B. D., Scott D. B. Isolation and characterization of transposon Tn5-induced symbiotic mutants of Rhizobium loti. J Bacteriol. 1985 Apr;162(1):335–343. doi: 10.1128/jb.162.1.335-343.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curello S., Ceconi C., Bigoli C., Ferrari R., Albertini A., Guarnieri C. Changes in the cardiac glutathione status after ischemia and reperfusion. Experientia. 1985 Jan 15;41(1):42–43. doi: 10.1007/BF02005863. [DOI] [PubMed] [Google Scholar]
- Galinski E. A., Pfeiffer H. P., Trüper H. G. 1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid. A novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur J Biochem. 1985 May 15;149(1):135–139. doi: 10.1111/j.1432-1033.1985.tb08903.x. [DOI] [PubMed] [Google Scholar]
- Klapwijk P. M., Scheulderman T., Schilperoort R. A. Coordinated regulation of octopine degradation and conjugative transfer of Ti plasmids in Agrobacterium tumefaciens: evidence for a common regulatory gene and separate operons. J Bacteriol. 1978 Nov;136(2):775–785. doi: 10.1128/jb.136.2.775-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan J. T., Long S. R. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6609–6613. doi: 10.1073/pnas.82.19.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankhurst C. E., Broughton W. J., Wieneke U. Transfer of an indigenous plasmid of Rhizobium loti to other rhizobia and Agrobacterium tumefaciens. J Gen Microbiol. 1983 Aug;129(8):2535–2543. doi: 10.1099/00221287-129-8-2535. [DOI] [PubMed] [Google Scholar]
- Robertson J. G., Warburton M. P., Farnden K. J. Induction of glutamate synthase during nodule development in lupin. FEBS Lett. 1975 Jul 15;55(1):33–37. doi: 10.1016/0014-5793(75)80950-1. [DOI] [PubMed] [Google Scholar]
- Schell J., Van Montagu M., De Beuckeleer M., De Block M., Depicker A., De Wilde M., Engler G., Genetello C., Hernalsteens J. P., Holsters M. Interactions and DNA transfer between Agrobacterium tumefaciens, the Ti-plasmid and the plant host. Proc R Soc Lond B Biol Sci. 1979 Apr 11;204(1155):251–266. doi: 10.1098/rspb.1979.0026. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]