Abstract
Background
The PROACT II trial showed that intra‐arterial thrombolysis (IAT) is effective for treatment of acute ischaemic stroke attributable to M1 and M2 segment occlusions. Incidence of symptomatic intracranial haemorrhage (sICH) was 10%.
Objective
: To evaluate the risk and predictors of sICH after IAT by using urokinase in a large number of patients presenting with the whole spectrum of cerebral vessel occlusions.
Methods
294 patients with stroke treated with intra‐arterial urokinase were retrospectively analysed. The risk of sICH as well as bleeding characteristics were assessed. Demographic and radiological data, time to treatment, urokinase dose, recanalisation rates, stroke aetiology and severity were analysed for predictors.
Results
sICH occurred in 14 of 294 (4.8%) patients. The median National Institute of Health Stroke Scale score of all patients was 15. All but one sICH were located in the infarcted brain tissue, and no sICH occurred in patients with peripheral vessel occlusions (M3 or M4 segments of the middle cerebral artery). Poor collaterals (p = 0.001), early signs of ischaemia on computed tomography (p = 0.003), higher urokinase dose (p = 0.019), lower recanalisation rate (p = 0.02) and higher diastolic blood pressure on admission (p = 0.04) were found to be correlated with sICH on univariate analysis. On multivariate analysis, poor collaterals (p = 0.004), urokinase dose (p = 0.021) and early signs on computed tomography (p = 0.026) remained predictors of sICH.
Conclusions
With regard to the whole spectrum of cerebral vessel occlusions, an incidence of <5% sICH after IAT is distinctly low. This result underlines the important role of IAT in the treatment of acute stroke.
The aim of treatment in acute ischaemic stroke is revascularisation as fast as possible. For this purpose, both intravenous thrombolysis (IVT) and intra‐arterial thrombolysis (IAT) have proved to be effective.1,2,3,4,5 The most devastating complication of both treatments is intracranial haemorrhage (ICH). ICH is categorised into haemorrhagic transformation, which is usually petechial and asymptomatic, and parenchymal haematomas without deterioration and those with clinical deterioration. Those with clinical deterioration are referred to as symptomatic ICH (sICH), which is associated with an increased mortality and occurs spontaneously in 0.6–4% of patients with ischaemic strokes. Thrombolysis increases the risk of sICH. Current literature reports wide ranges of incidence—for example, 3.3–21.2% for IVT and 0–14.3% for IAT.1,3,6,7,8,9,10,11,12,13,14
The largest IAT series was the PROACT II trial reporting on a defined subgroup of patients with stroke (n = 180) exclusively with M1 and M2 segment occlusions of the middle cerebral artery (MCA).3
This study was conducted to evaluate the risk of sICH in the whole spectrum of patients with large cerebral artery occlusions treated with IAT. Characteristics of patients with sICH were assessed and predictors analysed.
Patients and methods
Patients
This study includes all patients presenting with acute ischaemic stroke at the University of Berne, Berne, Switzerland, from December 1992 to March 2004 and who were treated with IAT using urokinase (n = 294, 133 women, mean age 60 years). Subgroups of this sample have been published in studies on other issues.4,5,15 Patients treated with urokinase application into the ophthalmic artery for retinal ischaemia were not included.16
Inclusion criteria for patients treated with IAT were as follows:
Clinical diagnosis of acute stroke established by a neurologist
Baseline National Institutes of Health Stroke Scale (NIHSS)17 score of at least 4 points, except for isolated hemianopia or aphasia
Exclusion of haemorrhage by cranial computed tomography or magnetic resonance imaging (MRI)
Cerebral angiography showing vessel occlusion correlated with neurological deficit
Time interval from symptom onset to beginning of IAT <6 h, except in basilar artery occlusion, where longer intervals were permitted
No individual clinical or laboratory findings advising against thrombolysis
For patients aged >75 years, that their general condition before stroke did not advise against it
Informed consent from patient or family was available.
Imaging and intervention
Effacement of sulci and obscuration of the normally hyperdense grey matter on initial computed tomography were considered to be early computed tomographic signs of cerebral infarction.18,19,20 Focal hyperdensity of a cerebral artery representing intraluminal clot was noted as a hyperdense artery sign.
Selective intra‐arterial digital subtraction angiography was carried out on a biplane, high‐resolution angiography system (Toshiba CAS 500, Tokyo, Japan) with a matrix of 1024×1024 pixels. For vessel contrast, Iopamidol (Iopamiro 300, Bracco, Milan, Italy) was used.
Generally, angiograms of the complete anterior and posterior cerebral circulation were acquired to assess collateral blood supply to the infarcted area. Collaterals were classified semiquantitively into two groups: poor, if none or minimal leptomeningeal collaterals were present; and good, if leptomeningeal anastomoses filled more than half of the occluded vessel territory.15
Diagnostic angiography was followed by superselective catheterisation of the occluded vessel using a microcatheter, mostly a Fast Tracker 18 (Boston Scientific Target, Fremont, California, USA). Occasionally, the thrombus was gently passed with the microcatheter or the microguidewire, mostly a Silver Speed 0.010 or 0.014 inch (MTI, Irvine, California, USA). The tip of the microcatheter was placed beside or near the proximal end of the thrombus, and urokinase (Urokinase 500 000 HS, Medac, Wedel, Germany) was injected manually. The applied dose ranged from 20 000 to 1 500 000 U (median dose 1 000 000 U). Normally, application of urokinase took 60–90 min, but was terminated if follow‐up angiograms taken during IAT showed vessel recanalisation. Additional percutaneous transluminal angioplasty (PTA) or stenting of the extracranial vessels was carried out in 25 patients.21,22 The follow‐up angiogram after completion of IAT was used for assessment of recanalisation, which was classified according to thrombolysis in myocardial infarction (TIMI) grades as follows: no recanalisation, TIMI grade 0; minimal recanalisation, TIMI grade 1; partial recanalisation, TIMI grade 2; and complete recanalisation, TIMI grade 3.23
Follow‐up
Patients were supervised at the intensive or intermediate care unit. Before publication of the results of the International Stroke Trial, patients received heparin in a dose doubling the activated thromboplastin time immediately after IAT. 24 After the International Stroke Trial, this protocol was changed and aspirin (250–500 mg) was given after IAT to 234 patients.
Follow‐up computed tomography or MRI was routinely carried out within 24 h after IAT or in the case of neurological deterioration, headaches or decline in consciousness. sICH was defined as parenchymal haematoma causing mass effect on computed tomography or MRI, with clinical deterioration defined as a 4‐point or greater increase in the NIHSS score, or a 1‐point deterioration in the level of consciousness.3
Outcome was assessed by a neurologist 3 months after IAT using the modified Rankin Scale (mRS).25 All sICHs that occurred during the first 3 months after IAT were counted.
Infarct aetiology was classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria.26 The following risk factors for stroke were assessed: history of hypertension (preadmission values >140/90 mm Hg or use of antihypertensive agents); types 1 and 2 diabetes (defined by preadmission history, raised level of glycated haemoglobin or venous plasma glucose concentration >7.0 mmol/l after an overnight fast on at least two separate occasions); current cigarette smoking; hyperlipidaemia (defined as a total venous plasma cholesterol concentration >5 mmol/l); and personal or family history of cardiovascular ischaemic event.
Patients were divided into groups with anterior or posterior circulation strokes. Patients with anterior circulation strokes were stratified according to their occlusion site into proximal (ICA, M1 and M2 segments of the MCA) and distal (M3 and M4 segments of the MCA) occlusion groups.
All data were continuously recorded at our stroke unit database and assessed in retrospect. The study design conforms with the guidelines of the local university's ethics committee and was conducted according to the revised Declaration of Helsinki of 1998.
Statistical analysis
Data were statistically analysed using SPSS V.10 for Macintosh statistical software. For comparisons the patients were divided into two groups: (1) patients with sICH were defined as the bleeding group and (2) patients without sICH served as the control group. Age is expressed as mean (standard deviation (SD)). NIHSS scores, time intervals, blood pressures and urokinase doses are expressed as median (interquartile range (IQR)).
The associations of sICH with age, NIHSS scores, time to treatment, blood pressure on admission, urokinase doses, TIMI grades and TOAST criteria were analysed using the Mann–Whitney U test. Between‐group comparisons of sex, vascular risk factors, positive personal or family history of cerebrovascular ischaemic events, treatment with aspirin or other antiplatelet agents or vitamin K antagonists before IAT, treatment with aspirin or heparin after IAT, presence of early computed tomographic signs, collaterals and occlusion site were determined using the two‐sided Fisher's exact test for 2×2 contingency table. Follow‐up mRS scores of the two groups were compared using the Mann–Whitney U test.
Owing to the small number of patients who had sICH, logistic regression analysis included only selected variables that were significant predictors on univariate analysis.
For univariate and multivariate analysis, values of p<0.05 were considered to be significant.
Results
In all, 294 patients (133 women, mean age 60 years) underwent IAT using urokinase for treatment of acute ischaemic stroke. The Median NIHSS score on admission was 15. Also, 14 (4.8%, 95% confidence interval (CI) 2.4% to 7.2%) patients developed sICH after treatment.
Group comparisons
Univariate analysis of demographic data and presenting clinical characteristics
Table 1 shows demographic data and presenting clinical characteristics, including vascular risk factors, NIHSS score, blood pressure on admission, and treatment with antithrombotics of patients with and without sICH. The two groups did not differ in terms of age, sex, stroke severity, prevalence of vascular risk factors, and preceding treatment with aspirin, clopidogrel and vitamin K antagonists. Stroke aetiologies according to the TOAST criteria were also not different. Diastolic blood pressure on admission was higher in patients with sICH (p = 0.04).
Table 1 Comparison of demographic data and vascular risk factors in the bleeding and non‐bleeding groups.
| Characteristic | sICH | No sICH | Total | p Value |
|---|---|---|---|---|
| Sex, n = 294 | ||||
| Men | 7 | 154 | 54.8% | 0.787* |
| Women | 7 | 126 | 45.2% | |
| Age, years (mean (SD)) | 63 (12) | 60 (13) | 60 (13) | 0.338† |
| NIHSS on admission | 16 (7.00) | 15 (8.75) | 15 (8.00) | 0.507† |
| Arterial hypertension, n = 293 | ||||
| Yes | 9 | 150 | 54.3% | 0.585* |
| No | 5 | 129 | 45.7% | |
| Hyperlipidaemia, n = 293 | ||||
| Yes | 3 | 98 | 34.5% | 0.393* |
| No | 11 | 181 | 65.5% | |
| Smoking, n = 293 | ||||
| Yes | 3 | 63 | 22.5% | 1.000* |
| No | 11 | 216 | 77.5% | |
| Family history of CVE, n = 226 | ||||
| Yes | 3 | 55 | 25.7% | 0.697* |
| No | 6 | 162 | 74.3% | |
| History of CVE, n = 294 | ||||
| Yes | 2 | 36 | 12.9% | 0.699* |
| No | 12 | 244 | 87.1% | |
| Diabetes mellitus, n = 293 | ||||
| Yes | 3 | 37 | 13.7% | 0.417* |
| No | 11 | 242 | 86.3% | |
| Blood pressure on admission (mm Hg) | n = 10 | n = 184 | n = 194 | |
| Systolic | 145 (65) | 145 (39) | 145 (40) | 0.832† |
| Diastolic | 92.5 (22.5) | 80 (20) | 80 (20) | 0.040† |
| Aspirin or clopidogrel before IAT, n = 198 | ||||
| Yes | 5 | 35 | 20.2% | 0.164* |
| No | 9 | 149 | 79.8% | |
| Vitamin K antagonist before IAT, n = 293 | ||||
| Yes | 1 | 6 | 2.4% | 0.293* |
| No | 13 | 273 | 97.6% |
CVE, cerebrovascular ischaemic event; IAT, intra‐arterial thrombolysis; sICH, symptomatic intracranial haemorrhage.
Numbers in parentheses are interquartile ranges, unless specified otherwise.
*Fisher's exact test.
†Mann–Whitney U test.
Univariate analysis of radiological and treatment characteristics
Table 2 summarises the imaging findings, IAT parameters, recanalisation rates and antithrombotic treatment after IAT in both groups. Poor leptomeningeal collaterals (odds ratio (OR) 9.35, 95% CI 2.04 to 41.67, p = 0.001), presence of early computed tomographic signs (OR 7.73, 95% CI 1.68 to 35.56, p = 0.003), overall dose of urokinase (p = 0.019) and lower recanalisation rate after IAT (p = 0.02) were found to be associated with sICH. Overall, partial or complete recanalisation (TIMI grades 2 and 3) was achieved in 70.3% of the 294 patients.
Table 2 Comparison of imaging findings, intra‐arterial thrombolysis parameters, antithrombotic treatment and recanalisation rates in the bleeding and non‐bleeding groups.
| Characteristic | sICH | No sICH | Total | p Value |
|---|---|---|---|---|
| Dense artery sign, n = 270 | ||||
| Yes | 6 | 109 | 42.6% | 0.767* |
| No | 6 | 149 | 57.4% | |
| Early CT signs of ischaemia, n = 280 | ||||
| Yes | 11 | 111 | 43.6% | 0.003* |
| No | 2 | 156 | 56.4% | |
| Occlusion site, n = 293 | ||||
| Anterior circulation | 13 | 235 | 86.4% | 0.703* |
| Posterior circulation | 1 | 44 | 13.6% | |
| ICA and MCA, n = 243 | ||||
| Proximal (ICA, M1 or M2) | 13 | 193 | 84.8% | 0.227* |
| Distal (M3 or M4) | 0 | 37 | 15.2% | |
| Collaterals, n = 287 | ||||
| Good | 2 | 166 | 58.5% | 0.001* |
| Poor | 12 | 107 | 41.5% | |
| Time from stroke onset to IAT, min | 247.5 (105) | 255 (101) | 255 (100) | 0.851† |
| Urokinase dose, U | 1 000 000 (0) | 1 000 000 (250 000) | 1 000 000 (250 000) | 0.019† |
| PTA/stent, n = 293 | ||||
| Yes | 1 | 24 | 8.5% | 1.000* |
| No | 13 | 255 | 91.5% | |
| TIMI grades | n = 14 | n = 276 | 0.020† | |
| 0 | 1 | 47 | 16.5% | |
| 1 | 8 | 30 | 13.1% | |
| 2 | 5 | 150 | 53.4% | |
| 3 | 0 | 49 | 16.9% | |
| Aspirin after IAT, n = 293 | ||||
| Yes | 9 | 225 | 79.9% | 0.167* |
| No | 5 | 54 | 20.1% | |
| Heparin after IAT, n = 294 | ||||
| Yes | 1 | 51 | 17.7% | 0.477* |
| No | 13 | 229 | 82.3% |
CT, computed tomography; IAT, intra‐arterial thrombolysis; ICA, internal carotid artery; M1–M4, segments of the middle carotid artery; MCA, middle cerebral artery; PTA, percutaneous transluminal angioplasty; sICH, symptomatic intracranial haemorrhage; TIMI, thrombolysis in myocardial infarction.
Numbers in parentheses are interquartile ranges.
*Fisher's exact test.
†Mann—Whitney U test.
Multivariate analysis
Logistic regression analysis with forward selection included factors that were significant on univariate analysis except for diastolic blood pressure on admission. This factor was removed from the model because of too many patients with missing data, which resulted in a reduction in the number of available patients and hence a decrease in the reliability of the multivariate analysis. In all, 13 patients with sICH and 260 patients without sICH could be included in the model (n = 273). For statistical purpose, OR associated with urokinase dose was calculated for an increase of 10 000 U in the dose of urokinase.
The following factors were found to be independent risk factors of sICH after IAT: (1) poor collaterals (p = 0.004, OR 11.38, 95% CI 2.16 to 60.12); (2) overall dose of urokinase (p = 0.021, OR 1.06, 95% CI 1.01 to 1.12); and (3) early computed tomographic signs of cerebral ischaemia (p = 0.026, OR 5.99, 95% CI 1.24 to 28.82). Recanalisation rate reached no significance after adjustment for these three factors.
Characteristics of patients with sICH
Table 3 summarises vessel occlusion sites, NIHSS scores, time intervals, urokinase doses, bleeding characteristics and outcome. Occlusion was always complete. No distal occlusions of M3 or M4 segments or isolated anterior or posterior cerebral artery infarctions were encountered.
Table 3 Patients with symptomatic intracranial haemorrhage.
| Patient no, sex, age (years) | NIHSS score | Occlusion site | IAT | Symptomatic intracranial haemorrhage | mRS‡ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Delay* (min) | Duration (min) | Dose† | TIMI grade | Delay (h) | Inside the infarct | Localisation | ||||
| 1, M, 57 | 19 | CTO, incl ACA, PCA | 210 | 105 | 1.25 | 1 | 1 | Yes | Lobar, BG, IVH | 6 |
| 2, F, 75 | 19 | CTO, incl PCA | 300 | 60 | 1 | 1 | 14 | Yes | Lobar, IVH | 6 |
| 3, M, 70 | 14 | CTO | 330 | 60 | 1 | 2 | 10 | Yes | BG, IVH, SAH | 6 |
| 4, F, 49 | 12 | M1+LS | 360 | 60 | 1 | 2 | 7 | Yes | Lobar, BG, IVH | 4 |
| 5, M, 67 | 19 | M1+LS | 300 | 60 | 1 | 2 | <24 | Yes | Lobar, BG, IVH, SAH | 5 |
| 6, F, 68 | 21 | M1+LS | 180 | 60 | 1 | 1 | 4 | Yes | Lobar, BG, IVH | 6 |
| 7, F, 36 | 13 | M1+LS | 215 | 60 | 1 | 0 | 48–72 | Yes | BG | 3 |
| 8, M, 69 | 19 | M1+LS | 270 | 60 | 1 | 1 | <24 | Yes | Lobar, BG, IVH | 6 |
| 9, M, 60 | 12 | M1 | 230 | 60 | 1 | 2 | 48–72 | Yes | Lobar | 2 |
| 10, F, 71 | 11 | M2 | 180 | 65 | 1 | 1 | 16 | Yes | Lobar, BG, IVH | 3 |
| 11, M, 73 | 12 | M2 | 255 | 50 | 1 | 1 | <24 | Yes | Lobar, IVH | 6 |
| 12, F, 70 | 12 | M2 | 20 | 60 | 0.75 | 2 | 5 | No | Lobar, IVH, SDH | 4 |
| 13, M, 74 | 18 | M2 | 240 | 50 | 1 | 1 | 7 | Yes | Lobar | 6 |
| 14, F, 43 | 35 | BA | 385 | 60 | 1 | 1 | <24 | Yes | Pons, IVH | 6 |
ACA, anterior cerebral artery; BA, basilar artery; BG, basal ganglia; CTO, carotid T occlusion; F, female; IVH, intraventricular hyperdensity; LS, lenticulostriate arteries; M, male; M1 and M2, segments of middle cerebral artery; mRS, modified Rankin Score; PCA, posterior cerebral artery; SAH, subarachnoidal haemorrhage; SDH, subdural haemorrhage.
*Time interval from symptom onset to start of IAT.
†×106 U urokinase.
‡Evaluation after 3 months.
One patient had been treated with high‐dose heparin before IAT because of vertebral artery dissection.
sICH occurred within 24 h after IAT in 12 patients and after 3 days in two patients. The shortest interval from IAT to haemorrhage was 1 h. Haemorrhage was localised inside the infarcted area in 13 patients and outside in one. MRI in this patient showed marked signs of cerebral microangiopathy and old microbleeds. Haemorrhage was multifocal in 12 patients and unilocular in two.
No ICH occurred during IAT and no procedure‐related artery dissection was noted. Table 4 shows the rates of sICH for the different vessel territories.
Table 4 Occlusion site and rate of symptomatic intracranial haemorrhage (n = 293).
| Cerebral vessel occlusion | Patients (n) | sICH (n) | Percentage |
|---|---|---|---|
| Internal carotid artery | 41 | 3 | 7.3 |
| M1 or M2 segment of MCA | 165 | 10 | 6.0 |
| M3 or M4 segment of MCA | 37 | 0 | 0 |
| Anterior cerebral artery | 5 | 0 | 0 |
| Posterior cerebral artery | 4 | 0 | 0 |
| Basilar artery | 41 | 1 | 2.4 |
MCA, middle cerebral artery; sICH, symptomatic intracranial haemorrhage.
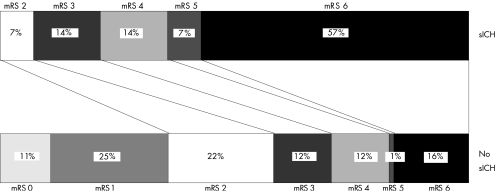
Figure 1 shows the outcome at 3 months. Three patients were lost to follow‐up. sICH was significantly correlated to a poor outcome reflected by higher mRS scores (p<0.001).
Figure 1 Modified Rankin Scale (mRS) scores of patients treated with intra‐arterial thrombolysis (n = 291) at 3 months follow‐up. sICH, symptomatic intracranial haemorrhage.
Discussion
sICH is the most feared adverse event of thrombolysis of acute ischaemic stroke and is associated with a mortality of 75–83%.12,13,14 In this large series of 294 IAT patients with a median NIHSS score of 15 on admission, 14 suffered sICH. Mortality after sICH was 57%; furthermore, 36% of the patients had severe disability after 3 months.
General findings
The incidence of sICH in this study (4.8%) was approximately half of that in the PROACT II trial (10%). This might be explained by several reasons:
Longer median time from symptom onset to treatment in PROACT II (318 v 255 min).
Higher median NIHSS scores of the patients in the PROACT II trial (17 v 15).
Higher mean age of the patients in the PROACT II trial (64 v 60 years).
A slightly lower recanalisation rate 2 h after initiation of IAT in PROACT II (66% TIMI grades 2 and 3 v 70% in our study). Points (1) and (4) indicate prolonged ischaemia in patients in the PROACT trial with a higher risk of ICH.
PROACT II prohibited mechanical maneouvres at the occlusion site, whereas our study permitted both passage of the thrombus with the microguidewire or microcatheter and injection of urokinase into the thrombus, presumably resulting in faster recanalisation and less flow of the thrombolytic to the basal ganglia.
Different thrombolytic drugs were used and the patients in the PROACT trial were given heparin.2,3,14
In contrast with the large studies on IVT reporting haematoma localisation outside the infarct in 20–35% of the bleeding patients, sICH after IAT in this and other series usually occurred in the area of the infarct.13,14,27 In our series only one sICH was located outside, in a patient with marked signs of cerebral microangiopathy and old microbleeds on MRI. Microangiopathy and microbleeds are discussed as risk factors for both spontaneous ICH and haemorrhage after thrombolysis.27,28,29,30 This observation points to a risk of haemorrhage in brain tissue not primarily affected by the stroke after IVT, which is smaller after IAT. Accordingly, the rate of sICH in this series was lower than in most studies on IVT.
Predictors of sICH
In this study, patients who bled were more likely to have poor collaterals, early computed tomographic signs of ischaemia, received a higher dose of urokinase, and achieved a lower recanalisation rate after IAT on univariate analysis. After multivariate analysis, poor collaterals, early computed tomographic signs of ischaemia and a higher urokinase dose remained as independent predictors for sICH.
Poor collaterals usually result in larger infarcts, which have been found to increase the risk of sICH after thrombotysis.6,13,31 Patients with such big infarcts were more likely to present with higher NIHSS scores on admission. In this series, NIHSS scores of all patients who bled were ⩾11. However, NIHSS score was not a risk factor for sICH. A potential explanation is that irreversibly damaged tissue rather than the severity of the neurological deficit on admission is the predisposing factor for sICH. Irreversible tissue damage is visualised as early infarct signs on computed tomography.19,32 Such signs, rather than the NIHSS score, were found to be an independent predictor of sICH in this series.
The urokinase dose was found to be associated with sICH in keeping with studies showing a higher bleeding rate with increasing dose of thrombolytics.3 A dose of 1 000 000 U urokinase was exceeded only in 10 of 294 patients and might contribute to the overall low rate of sICH in our series. In all, 12 of the 14 sICHs occurred in the first 24 h after IAT, which relates to the pharmacology of urokinase. Although plasma half life of urokinase is <20 min, plasmin activation persists for 12–24 h with impact on the extracellular matrix and activation of metalloproteinases potentially resulting in blood–brain barrier damage.33,34,35
Higher diastolic blood pressure on admission was predictive of sICH on univariate analysis. This has been found by authors looking at blood pressure on admission and outcome in larger series of patients with stroke treated with IVT as well.6,9
Finally, lower recanalisation rates after IAT were associated with sICH on univariate analysis. No patient who bled had a complete recanalisation as seen on digital subtraction angiography immediately after IAT. This indicates that early reperfusion is unlikely to be a risk factor for sICH. However, we cannot rule out delayed clot lysis and recanalisation contributing to the risk of sICH.
To summarise, several predictors of sICH after IAT were found. However, our data do not advise against IAT with urokinase for treatment of patients with acute ischaemic stroke presenting with such risk factors, because these patients tend to have a poor spontaneous outcome even without sICH.
Limitations
Although this study includes a large number of patients treated with IAT, the number of those who had sICH is low, reducing the power of the multivariate analysis. Different doses of urokinase were used depending on the individual patient treated. Blood pressures on admission were available in only two thirds of the patients in this retrospective study and consequently were excluded from the multivariate analysis. Blood pressure values after IAT were not recorded in our stroke database.
Conclusions
An incidence of <5% sICH after IAT for the whole spectrum of patients with cerebral vessel occlusion is very low. This result underlines the important role of IAT in the treatment of patients with acute stroke.
Acknowledgements
We thank Pietro Ballinari, PhD, for statistical advice.
Abbreviations
IAT - intra‐arterial thrombolysis
IQR - interquartile range
IVT - intravenous thrombolysis
MCA - middle cerebral artery
MRI - magnetic resonance imaging
mRS - modified Rankin Scale
NIHSS - National Institutes of Health Stroke Scale
PTA - percutaneous transluminal angioplasty
sICH - symptomatic intracranial haemorrhage
TIMI - thrombolysis in myocardial infarction
TOAST - Trial of Org 10172 in Acute Stroke Treatment
Footnotes
Competing interests: None.
References
- 1.The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 19953331581–1587. [DOI] [PubMed] [Google Scholar]
- 2.del Zoppo G J, Higashida R T, Furlan A J.et al PROACT: a phase II randomized trial of recombinant pro‐UK by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in Acute Cerebral Thromboembolism. Stroke 1998294–11. [DOI] [PubMed] [Google Scholar]
- 3.Furlan A, Higashida R, Wechsler L.et al Intra‐arterial proUK for acute ischemic stroke. The PROACT II study: a randomized controlled trial, Prolyse in Acute Cerebral Thromboembolism. JAMA 19992822003–2011. [DOI] [PubMed] [Google Scholar]
- 4.Arnold M, Schroth G, Nedeltchev K.et al Intra‐arterial thrombolysis in 100 patients with acute stroke due to middle cerebral artery occlusion. Stroke 2002331828–1833. [DOI] [PubMed] [Google Scholar]
- 5.Arnold M, Nedeltchev K, Schroth G.et al Clinical and radiological predictors of recanalisation and outcome of 40 patients with acute basilar artery occlusion treated with intra‐arterial thrombolysis. J Neurol Neurosurg Psychiatry 200475857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larrue V, von Kummer R R, Muller A.et al Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European‐Australasian Acute Stroke Study (ECASS II). Stroke 200132438–441. [DOI] [PubMed] [Google Scholar]
- 7.Albers G W, Bates V E, Clark W M.et al Intravenous tissue‐type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA 20002831145–1150. [DOI] [PubMed] [Google Scholar]
- 8.The Multicenter Acute Stroke Trial—Europe Study Group Thrombolytic therapy with streptokinase in acute ischemic stroke. N Engl J Med 1996335145–150. [DOI] [PubMed] [Google Scholar]
- 9.Gilligan A K, Markus R, Read S.et al Baseline blood pressure but not early computed tomography changes predicts major hemorrhage after streptokinase in acute ischemic stroke. Stroke 2002332236–2242. [DOI] [PubMed] [Google Scholar]
- 10.Zeumer H, Freitag H J, Zanella F.et al Local intra‐arterial fibrinolytic therapy in patients with stroke: urokinase versus recombinant tissue plasminogen activator (r‐TPA). Neuroradiology 199335159–162. [DOI] [PubMed] [Google Scholar]
- 11.Nakano S, Iseda T, Kawano H.et al Parenchymal hyperdensity on computed tomography after intra‐arterial reperfusion therapy for acute middle cerebral artery occlusion: incidence and clinical significance. Stroke 2001322042–2048. [DOI] [PubMed] [Google Scholar]
- 12.Fiorelli M, Bastianello S, von Kummer R.et al Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3‐month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 1999302280–2284. [DOI] [PubMed] [Google Scholar]
- 13.The NINDS t‐PA Stroke Study Group Intracerebral hemorrhage after intravenous t‐PA therapy for ischemic stroke. Stroke 1997282109–2118. [DOI] [PubMed] [Google Scholar]
- 14.Kase C S, Furlan A J, Wechsler L R.et al Cerebral hemorrhage after intra‐arterial thrombolysis for ischemic stroke: the PROACT II trial. Neurology 2001571603–1610. [DOI] [PubMed] [Google Scholar]
- 15.Gonner F, Remonda L, Mattle H.et al Local intra‐arterial thrombolysis in acute ischemic stroke. Stroke 1998291894–1900. [DOI] [PubMed] [Google Scholar]
- 16.Arnold M, Koerner U, Remonda L.et al Comparison of intra‐arterial thrombolysis with conventional treatment in patients with acute central retinal artery occlusion. J Neurol Neurosurg Psychiatry 200576196–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brott T, Adams H P, Jr, Olinger C P.et al Measurements of acute cerebral infarction: a clinical examination scale. Stroke 198920864–870. [DOI] [PubMed] [Google Scholar]
- 18.Tomura N, Uemura K, Inugami A.et al Early CT finding in cerebral infarction: obscuration of the lentiform nucleus. Radiology 1988168463–467. [DOI] [PubMed] [Google Scholar]
- 19.Truwit C L, Barkovich A J, Gean‐Marton A.et al Loss of the insular ribbon: another early CT sign of acute middle cerebral artery infarction. Radiology 1990176801–806. [DOI] [PubMed] [Google Scholar]
- 20.von Kummer R, Meyding‐Lamade U, Forsting M.et al Sensitivity and prognostic value of early CT in occlusion of the middle cerebral artery trunk. Am J Neuroradiol 1994159–15. [PMC free article] [PubMed] [Google Scholar]
- 21.Nedeltchev K, Remonda L, Do D D.et al Acute stenting and thromboaspiration in basilar artery occlusions due to embolism from the dominating vertebral artery. Neuroradiology 200446686–691. [DOI] [PubMed] [Google Scholar]
- 22.Nedeltchev K, Brekenfeld C, Remonda L.et al Stenting of the internal carotid artery in acute stroke: preliminary results of 25 patients. Radiology 20052371029–1037. [DOI] [PubMed] [Google Scholar]
- 23.The TIMI Study Group The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N Engl J Med 1985312932–936. [DOI] [PubMed] [Google Scholar]
- 24.The International Stroke Trial Collaborative Group The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet 19973491569–1581. [PubMed] [Google Scholar]
- 25.Van Swieten J C, Koudstaal P J, Visser M C.et al Interobserver agreement for the assessment of handicap in stroke patients. Stroke 198819604–607. [DOI] [PubMed] [Google Scholar]
- 26.Adams H P, Jr, Bendixen B H, Kappelle L J.et al Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 19932435–41. [DOI] [PubMed] [Google Scholar]
- 27.Larrue V, von Kummer R, del Zoppo G.et al Hemorrhagic transformation in acute ischemic stroke. Potential contributing factors in the European Cooperative Acute Stroke Study. Stroke 199728957–960. [DOI] [PubMed] [Google Scholar]
- 28.del Zoppo G J, von Kummer R, Hamann G F. Ischaemic damage of brain microvessels: inherent risks for thrombolytic treatment in stroke. J Neurol Neurosurg Psychiatry 1998651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fazekas F, Kleinert R, Roob G.et al Histopathologic analysis of foci of signal loss on gradient‐echo T2*‐weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy‐related microbleeds. Am J Neuroradiol 199920637–642. [PMC free article] [PubMed] [Google Scholar]
- 30.Nighoghossian N, Hermier M, Adeleine P.et al Old microbleeds are a potential risk factor for cerebral bleeding after ischemic stroke: a gradient‐echo T2*‐weighted brain MRI study. Stroke 200233735–742. [DOI] [PubMed] [Google Scholar]
- 31.von Kummer R, Hacke W. Safety and efficacy of intravenous tissue plasminogen activator and heparin in acute middle cerebral artery stroke. Stroke 199223646–652. [DOI] [PubMed] [Google Scholar]
- 32.Kucinski T, Majumder A, Knab R.et al Cerebral perfusion impairment correlates with the decrease of CT density in acute ischaemic stroke. Neuroradiology 200446716–722. [DOI] [PubMed] [Google Scholar]
- 33.Pfefferkorn T, Staufer B, Liebetrau M.et al Plasminogen activation in focal cerebral ischemia and reperfusion. J Cereb Blood Flow Metab 200020337–342. [DOI] [PubMed] [Google Scholar]
- 34.Wagner S, Hamann G F. Experimental microvascular and clotting changes—significance for acute stroke therapy. Nervenarzt 200374123–132. [DOI] [PubMed] [Google Scholar]
- 35.Koshelnick Y, Ehart M, Stockinger H.et al Mechanisms of signaling through urokinase receptor and the cellular response. Thromb Haemost 199982305–311. [PubMed] [Google Scholar]