Abstract
The pattern of reoccurrence of symptoms after discontinuation of deep brain stimulation (DBS) has not been systematically studied in dystonia. Eight patients (mean age (SD) 53.8 (14.4) years) with segmental dystonia at a mean follow‐up of 11.3 (4.2) months were studied after implantation of bilateral DBS electrodes in the internal globus pallidus using a standard video protocol and clinical rating scales, immediately and at 2 and 4 h after switching off DBS. Dystonic signs returned sequentially, with a rapid worsening of phasic and a slower worsening of tonic dystonic components. In all patients, phasic dystonic features appeared within a few minutes, whereas the tonic elements of dystonia reoccurred with a more variable delay. Differential clinical effects when withdrawing DBS might reflect its influence on different pathophysiological mechanisms in dystonia.
Chronic high‐frequency deep brain stimulation (DBS) has replaced radiofrequency lesioning in functional stereotactic surgery for treatment of dystonia within the past few years.1,2,3,4,5 Despite the unsolved debate about its primary mechanisms of action–that is, synaptic depression, synaptic inhibition, depolarisation blockade, changes in local blood flow and stimulation‐induced modulation of pathological network activity6,7–the option of postsurgical modification and optimisation of stimulation settings widens the indications for DBS. Pallidal DBS has been used for different manifestations of dystonia.1,2,3,4,5,8 Consistent improvement of the movement disorder and of associated disability has been shown, but little is known about the manifestation of dystonia without stimulation in patients with long‐term DBS. In contrast with Parkinson's disease, “On” and “Off” stimulation studies are uncommon in patients with dystonia.9 Although several genetic forms of dystonia could be linked to specific clinical patterns, the exact pathophysiological mechanisms in dystonia are poorly understood.
Improvement of dystonia with DBS occurs in a delayed and progressive manner.10 Recently, a study on the reappearance of clinical signs after discontinuation of DBS in Parkinson's disease has shown differential effects on the cardinal signs.11 To further elucidate the mechanisms of reoccurrence of different components of dystonia–that is, phasic dystonia (spasms and abnormal movements including dystonic tremor) and tonic dystonia (sustained muscle contractions with fixed abnormal postures)–we studied motor patterns after discontinuation of DBS.
Patients and methods
Eight consecutive adult patients (four men, four women) with severe segmental dystonia, who did not sufficiently benefit from prior medical treatment or local botulinum toxin treatment, were selected for chronic DBS and were included in this study (table 1).
Table 1 Patient characteristics.
| Patient number | Age (years) | Sex | Duration of dystonia (years) | Clinical characteristics and sites of dystonia | Preoperative treatment | Postoperative treatment |
|---|---|---|---|---|---|---|
| 1 | 39 | M | 10 | T>P neck, arms | None | None |
| 2 | 35 | F | 1 | T neck, arms | None | None |
| 3 | 62 | M | 5 | T>P face, neck, larynx, arms | None | None |
| 4 | 67 | F | 8 | P>T face, neck, larynx, arms, upper trunk | Metixene 15 mg/day, clonazepam 1.5 mg/day | Clonazepam 1.5 mg/day |
| 5 | 66 | F | 11 | P>T face, neck, larynx, arms, upper trunk | Trihexyphenidyl 6 mg/day, zolpidem 5 mg/day | Zolpidem 17.5 mg/day |
| 6 | 68 | M | 13 | P>T neck, arms | None | None |
| 7 | 56 | F | 2 | P>T face, neck, arms | Trihexyphenidyl 10 mg/day, doxepin 50 mg/day, tolperisone 150 mg/day | Tolperisone 100 mg/day |
| 8 | 37 | M | 20 | T>P face, neck, larynx, arms | None | None |
Face, oromandibular dystonia or blepharospasm; F, female; M, male; P, phasic dystonia; T, tonic dystonia
Exclusion criteria were psychiatric disorders, such as dementia (defined as Mini Mental State Examination score <25), major depression, psychosis or substance misuse, and pathological findings in cerebral magnetic resonance imaging, including cerebral atrophy or focal lesions. All patients had regular follow‐up visits in our movement disorders clinic. Informed consent was obtained for the surgery and the scheduled follow‐up examinations. Switching off neurostimulation was part of the routine periprocedural protocol. The procedures used in this study were approved by the local ethics committee in the frame of other studies of our group, and in agreement with the ethics committee no formal approval was obtained for this study. The patients' mean (standard deviation (SD)) age at surgery was 53.8 (14.4) years (range 35–68 years). Symptoms of dystonia had been present for 8.8 (6.2) years (range 1–20 years). The mean (SD) follow‐up interval for this study was 11.3 (4.2) months.
Concepts and techniques of the surgical procedure for DBS have been described in detail previously.8,12 All patients underwent simultaneous bilateral DBS of the globus pallidus internus (GPi). Quadripolar electrodes (3387 Medtronic, Minnesota, USA) were implanted in the posteroventral lateral GPi, guided by computed tomography‐stereotactic surgery and microelectrode recording, and connected to bilateral implantable pulse generators (Soletra 7426). Stimulation settings were stable for 2.8 (3.4) months. Usually, a bipolar configuration was chosen. On the right side, mean stimulation settings were voltage 3.8 (0.7) V, pulse width 210 µs and frequency 130 Hz. For the left side, voltage was 3.9 (0.6) V, pulse width 210 µs and frequency 130 Hz. Three patients received anticholinergic treatment at the time of surgery, and they discontinued treatment within 6 months after surgery.
Preoperative scores were used as baseline evaluation for each patient. For this study, clinical scores were assessed in the following conditions: with the stimulation on (S‐On), within the first 5 min after switching off DBS (S‐Off/0h), and at 2 and 4 h after switching off DBS (S‐Off/2h and S‐Off/4h). The standard protocol included the Unified Dystonia Rating Scale (UDRS), the Burke–Fahn–Marsden Dystonia Movement Scale (BFM), the Global Dystonia Rating Scale (GDS)13 and a standard video protocol. All examinations were performed by EG and CB, and videos were reassessed by the whole group until agreement on the scoring was achieved. We defined a tonic pattern of dystonia in the presence of more or less stable dystonic postures that might be exacerbated by activity but with only rare twitching movements and without tremulous or irregular jerking elements; by contrast, a phasic pattern displayed more or less continuous dystonic movements with intermittent sustained dystonic postures and also with tremulous or irregular jerking elements for more than half of the time during the standard video documentation.
Statistical analysis used non‐parametric tests for independent samples (Mann–Whitney U test) or multiple paired variables (Friedman test) as applicable. The statistical software system SPSS V.13 was used for all analyses.
Results
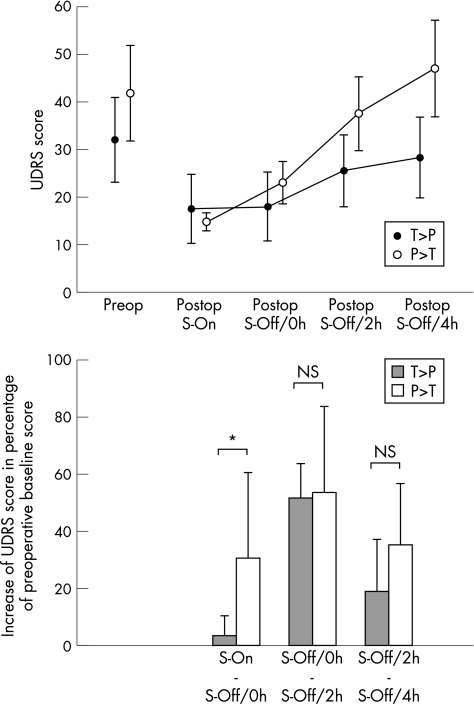
DBS showed a beneficial effect in all patients; there were no surgery‐related complications. The preoperative mean (SD) UDRS score was 36.9 (18.3), the mean (SD) BFM score was 35.6 (22.3) and the mean (SD) GDS score was 29.3 (25.7). The postoperative UDRS score at the time of the study decreased by a mean (SD) of 55.7% (15.3%; UDRS score 16.1 (9.9)), the BFM score by a mean (SD) of 60.6% (25%; BFM score 13.1 (13.6)) and the GDS score by a mean (SD) of 66.5% (10.3%; GDS score 10.1 (6.9)). Details of clinical outcome and long‐term follow‐up will be published elsewhere. Switching off DBS was associated with a reoccurrence of presurgical dystonic movement patterns in all patients (UDRS, BFM and GDS changed significantly over time; Friedman test, p<0.001). Clinically and as reflected by the changes of the three dystonia rating scales between S‐On and S‐Off/0h, discontinuation of DBS led to an early effect in the group of patients with predominantly phasic dystonia as compared with the group of patients with predominantly tonic dystonia (p<0.05 in UDRS and BFM scores, not significant p = 0.081 in GDS). Subsequently, both groups progressed towards the preoperative state at S‐Off/2h and S‐Off/4h (fig 1).
Figure 1 Top: Unified Dystonia Rating Scale (UDRS) score (mean (SEM)) preoperatively, and postoperatively at S‐On, S‐Off/0h, S‐Off/2h and S‐Off/4h. Black filled circles, means of patients with predominantly tonic (T) dystonia (T>P; n = 4); open circles, means of patients with predominantly phasic (P) dystonia (P>T; n = 4). Bottom: Stepwise increments of UDRS in percentage of preoperative baseline score for patients with predominantly tonic (T) dystonia (T>P; n = 4; filled bars) and with predominantly phasic dystonia (P>T; n = 4; open bars). *Significance at p = 0.05; NS, not significant.
Two videos of exemplary patients, one with predominantly tonic and the other with predominantly phasic dystonia are provided as supplemental material (http://jnnp.bmjjournals.com/supplemental).
Discussion
The occurrence of phasic and tonic components in dystonia is well known. Thus far, their differentiation has barely been considered relevant for diagnosis or treatment. Increasing experience with DBS in dystonia gave us the impression of differential effects on the different components of dystonia. To our knowledge, this is the first study that systematically investigates the effects of withdrawal of chronic DBS in patients with dystonia. Although it has been speculated that pallidal DBS might induce prolonged effects owing to neuroplastic changes, others have thought that discontinuation of DBS could result in an immediate medical emergency.5,10
After a stable period of stimulation, at approximately 1 year after surgery, all patients in our study had benefited from chronic DBS. After switching off DBS, the mean scores of BFM, UDRS and GDS asymptotically returned to the preoperative levels within 4 h. A clear difference in the reappearance of phasic and tonic dystonic movements became obvious: abnormal phasic movements recurred immediately, whereas abnormal tonic postures recurred more gradually. This pattern was independent of the sites of dystonia–that is, axial versus appendicular. Owing to the need to limit discomfort from switching off DBS, we restricted the time window to 4 h, despite which a stable state of dystonia might not have been reached in every patient.
The differential pattern of reoccurrence of dystonia reminds us of the withdrawal of chronic DBS in patients with Parkinson's disease. Recently, a sequential pattern of return of parkinsonian signs was found, with a rapid worsening of tremor and a delayed worsening of bradykinesia and rigidity.11
Obviously, the phasic and tonic components of dystonia are not independent of each other. With regard to the effects of chronic DBS, however, it seems that they are modulated by different mechanisms. The rapid effect on phasic movements is presumably associated with a direct change in electrical neurotransmission within basal ganglia loops involving the GPi. The more sustained effect on tonic abnormal postures after switching off DBS could account for slower pathophysiological processes, such as altered long‐term potentiation and cellular signalling via second messenger systems–not only locally but also in distant network relays.5,6,14 Improvement of dystonia with chronic DBS is probably due to disruption of altered pallidal activity, resulting in restoration of cortical and brain stem function.14,15
Similarly, the different patterns of reoccurrence of dystonia might be related to distinct anatomical networks.
Although the exact mechanisms of action of pallidal DBS in dystonia remain unknown, the results of our study indicate that there are different pathomechanisms subserving the phenomenology of dystonic movements or postures. From a clinical point of view, it seems that unexpectedly switching off chronic stimulation is more likely to result in a medical emergency in those patients in whom phasic dystonic movements prevail.
To view supplementary videos, see http://jnnp.bmj.com/supplemental
Copyright © 2007 BMJ Publishing Group Ltd
Supplementary Material
Acknowledgements
We thank Joe Jankovic, MD, Houston, Texas, and Cynthia Comella, MD, Chicago, Illinois, for providing us with the Unified Dystonia Rating Scale. We are grateful to Jose Rodrigues, Mannheim, Germany, for excellent videographic support.
Abbreviations
BFM - Burke–Fahn–Marsden Dystonia Movement Scale
DBS - deep brain stimulation
GDS - Global Dystonia Rating Scale
GPi - globus pallidus internus
UDRS - Unified Dystonia Rating Scale
Footnotes
Competing interests: Joachim K Krauss is a consultant to Medtronic, Minneapolis.
To view supplementary videos, see http://jnnp.bmj.com/supplemental
References
- 1.Krauss J K, Pohle T, Weber S.et al Bilateral stimulation of globus pallidus internus for treatment of cervical dystonia. Lancet 1999354837–838. [DOI] [PubMed] [Google Scholar]
- 2.Loher T J, Pohle T, Krauss J K. Functional stereotactic surgery for treatment of cervical dystonia: review of the experience from the lesional era. Stereotact Funct Neurosurg 2004821–13. [DOI] [PubMed] [Google Scholar]
- 3.Coubes P, Cif L, El Fertit H.et al Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia: long‐term results. J Neurosurg 2004101189–194. [DOI] [PubMed] [Google Scholar]
- 4.Vidailhet M, Vercueil L, Houeto J L.et al Bilateral deep‐brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 2005352459–467. [DOI] [PubMed] [Google Scholar]
- 5.Krauss J K, Yianni J, Loher T J.et al Deep brain stimulation for dystonia. J Clin Neurophysiol 20042118–30. [DOI] [PubMed] [Google Scholar]
- 6.McIntyre C C, Savasta M, Walter B L.et al How does deep brain stimulation work? Present understanding and future questions. J Clin Neurophysiol 20042140–50. [DOI] [PubMed] [Google Scholar]
- 7.Dostrovsky J O, Lozano A M. Mechanisms of deep brain stimulation. Mov Disord 200217(Suppl 3)S63–S68. [DOI] [PubMed] [Google Scholar]
- 8.Krauss J K, Loher T J, Pohle T.et al Pallidal deep brain stimulation in patients with cervical dystonia and severe cervical dyskinesias with cervical myelopathy. J Neurol Neurosurg Psychiatry 200272249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wöhrle J C, Weigel R, Grips E.et al Risperidone‐responsive segmental dystonia and pallidal deep brain stimulation. Neurology 200361546–548. [DOI] [PubMed] [Google Scholar]
- 10.Bittar R G, Yianni J, Wang S.et al Deep brain stimulation for generalised dystonia and spasmodic torticollis. J Clin Neurosci 20051212–16. [DOI] [PubMed] [Google Scholar]
- 11.Temperli P, Ghika J, Villemure J G.et al How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology 20036078–81. [DOI] [PubMed] [Google Scholar]
- 12.Krauss J K, Grossman R G. Principles and techniques of movement disorders surgery. In: Krauss JK, Jankovic J, Grossman RG, eds. Surgery for Parkinson's disease and movement disorders. Philadelphia, PA: Lippincott, Williams & Wilkins, 200174–109.
- 13.Comella C L, Leurgans S, Wuu J.et al Rating scales for dystonia: a multicenter assessment. Mov Disord 200318303–312. [DOI] [PubMed] [Google Scholar]
- 14.Vitek J L. Pathophysiology of dystonia: a neuronal model. Mov Disord 200217(Suppl 3)S49–S62. [DOI] [PubMed] [Google Scholar]
- 15.Silberstein P, Kuhn A A, Kupsch A.et al Patterning of globus pallidus local field potentials differs between Parkinson's disease and dystonia. Brain 20031262597–2608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.