Abstract
Background
Botulinum toxin type A (BoNT‐A) has become the treatment of choice for most types of focal dystonia.
Objective
To investigate the efficacy of BoNT‐A injections in patients with writer's cramp in a double‐blind, randomised, placebo‐controlled trial and to evaluate the follow‐up results.
Methods
Forty participants were randomised to treatment with either BoNT‐A or placebo injections in two sessions. Trial duration was 12 weeks. The primary outcome measure was the patients' choice to continue with the treatment, despite its possible disadvantages. Secondary outcome measures included several clinical rating scales on the levels of impairment and disability. Assessments were made at baseline and 2 months (secondary outcomes) and 3 months (primary outcome). Duration of follow‐up was 1 year.
Results
39 patients completed the trial. Fourteen of 20 patients (70%) receiving BoNT‐A reported a beneficial effect and chose to continue treatment, versus 6 of 19 patients (31.6%) in the placebo group (p = 0.03). The changes on most of the clinical rating scales were significantly in favour of BoNT‐A. Side effects reported were hand weakness, which was mostly mild and always transient, and pain at the injection site. After 1 year, 20 of 39 patients were still under treatment with a positive effect.
Conclusion
Treatment with BoNT‐A injections led to a significantly greater improvement compared with placebo, according to patients' opinion and clinical assessment scales. Weakness in the hand is an important side effect of BoNT‐A injections, but despite this disadvantage, most patients preferred to continue treatment. About 50% of our patients were still under treatment after 1 year.
Writer's cramp is a task‐specific, focal hand dystonia. It is characterised by involuntary, repetitive or sustained contractions of finger, hand or arm muscles that occur during writing and produce abnormal postures or movements that interfere with normal handwriting.1,2,3,4 Two categories are recognised: simple writer's cramp, in which dystonic posturing of the hand and arm occurs only during writing, and complex or dystonic writer's cramp, in which the condition manifests also during other manual tasks and sometimes with spontaneous abnormal posturing.1,2,5 In most patients, no specific cause can be identified. Although the prevalence is relatively low, varying from 3 to 7/100 000,6,7,8 writer's cramp may be responsible for considerable morbidity in terms of working impairment, pain, embarrassment, low self‐esteem and poor social interaction.
Therapeutic recommendations have included physical treatment, postural and writing re‐education exercises, relaxation techniques, hypnosis, biofeedback, use of special writing devices, acupuncture and transcranial magnetic stimulation, but most of the patients do not obtain satisfactory and sustained benefit.9,10,11,12 Some patients learn to write with their non‐dominant hand, but there is a 25% chance that this hand will become afflicted with the same problem.13 Drug treatment has been disappointing so far.3,9,14 The use of splints or braces and constraint‐induced movement treatment may occasionally be helpful, but it is not clear if they produce sustained relief.15,16,17 There is presently only limited experience with stereotactic neurosurgical procedures for focal hand dystonia.18,19 The treatment of dystonic syndromes such as blepharospasm and cervical dystonia has been much improved by the introduction of botulinum toxin as a therapeutic agent.20,21 When botulinum toxin is injected into muscles, the toxin produces local chemodenervation by interfering with the release of acetylcholine from the presynaptic nerve terminal.4 However, there are also several drawbacks. Firstly, the effects of botulinum toxin type A (BoNT‐A) are not permanent, lasting for only approximately 3 months; thus, regular injections are required. Secondly, inconvenient muscle weakness interfering with other non‐writing activities may occur.22 Regarding the treatment of writer's cramp, three randomised, double‐blind, placebo‐controlled studies have been undertaken, however, with small numbers of patients, different methods and inconclusive results.23,24,25
We performed a randomised, double blind, placebo‐controlled trial in 40 patients with writer's cramp, to assess whether the benefits of BoNT‐A treatment outweigh its disadvantages. The trial duration was 12 weeks and thereafter patients were followed for 1 year.
Methods
Participants
Patients were prospectively recruited from January 2001 to October 2003. They were eligible if they had signs and symptoms of idiopathic writer's cramp and had not benefited from BoNT‐A before. The aim was to include as many BoNT‐A naïve patients as possible. Patients were excluded if they were <18 years, were pregnant, and if they had multifocal, generalised or secondary dystonia, coagulation disorders or if the duration of illness was <1 year. Patients with marked writer's tremor were not included. Wilson's disease was excluded for all patients by caeruloplasmin and copper blood tests. The study was performed on an outpatient basis. Potentially suitable patients were referred by other clinicians, identified by the local investigator or self‐referred as a result of publicity about the study. Patients were invited to attend for screening 2–4 weeks before the start of treatment. Diagnosis of writer's cramp was based on the typical clinical presentation, including observation of handwriting and classified as simple or complex writer's cramp.1 All assessments were made by JJMK, who remained blind to the treatment allocation. The study protocol was approved by the local ethics committee at the Academic Medical Centre, University of Amsterdam (Amsterdam, The Netherlands). All patients received written information and gave their informed consent. Patients were randomised to treatment with either BoNT‐A injections or placebo injections. We allocated the patient a trial number and then forwarded relevant details to the central trial pharmacy. Randomisation was accomplished by an independent statistician using a computer program that allowed stratification according to the type of writer's cramp (simple v complex). Throughout the study, the list of treatment allocation codes was kept at the central trial pharmacy.
Interventions
Owing to individual differences in dose response, patients were treated in two sessions—the first after baseline assessment and the second after 1 month—unless patients were satisfied with the improvement after the first treatment session. In that case no injections were administered at the second session. If patients had no response to the first injections, the dose was doubled at the second session. In the case of insufficient response or muscle weakness, the injection dose and, if necessary, injection site were adjusted at the second session. This strategy was chosen to optimise the treatment effect. Concurrent treatment was not changed during the trial. Trial treatment consisted of BoNT‐A injections or placebo injections. Freeze‐dried BoNT‐A (Dysport, Ipsen Biopharma, Wrexham, UK) was diluted to 20 mouse units per 0.1 ml of 0.9% sterile saline by an independent pharmacist and aspirated in 1‐ml syringes. Placebo injections consisted of an equivalent volume of 0.9% sterile saline. BoNT‐A or saline was injected under simultaneous electromyographic recording into selected muscles using a hollow, Teflon‐coated, 27‐gauge needle. All injections were given by the same investigator (JHTMK), who remained blind to the treatment allocation and was not involved in the assessments. Muscles were selected for injection according to the pattern of movements and visible or palpable hypertonia. The number of muscles injected varied accordingly. The volume of fluid per injection site was dependent on the type of muscle. At the first visit, finger flexor muscles were injected with 60 IU (0.3 ml) per fascicle, finger extensors with 10–15 IU (0.05–0.075 ml) per fascicle, wrist flexors with 60–100 IU (0.3–0.5 ml) and wrist extensors with 30–40 IU (0.15–0.2 ml).
Assessment
All patients were assessed before treatment (baseline), after 4, 8 and 12 weeks by the same blinded investigator (JJMK).
The primary outcome measure was the patient's answer to the following question: considering all advantages and disadvantages of this treatment, is the improvement such that you wish to continue this treatment or not? We chose this primary outcome measure because it takes into consideration not only the improvement in dystonia symptoms but also the implications of hand weakness for the patient's daily activities and other possible disadvantages of the treatment. Assessment of the primary outcome was made at week 12.
Secondary outcome measures included the following rating scales: (1) visual analogue scale (VAS)26 for handwriting; (2) symptom severity scale (SSS)27; (3) functional status scale (FSS)27; (4) writer's cramp rating scale (WCRS)28; and (5) writing speed.
The VAS is a self‐assessment scale drawn by the patients on a 10‐cm line, on which 0 indicates the worst possible situation and 10 the best possible situation.26
The SSS consists of 10 questions that evaluate direct and indirect disease manifestations. Each answer is scored from 1 to 4 or 5 points to measure the severity specific to each region, and total score ranges from 10 (best possible) to 43 (worst possible).27
The FSS is a 12‐item disability scale that comprises an assessment of performances of daily activities that may possibly be affected by focal hand dystonia or hand weakness. A score ranging from 0 to 3 (no, mild, moderate, severe disability) is generated for each of 12 dimensions covering functional status.27 Total score ranges from 0 (best possible) to 36 (worst possible).
The SSS and FSS, which were originally designed for carpal tunnel syndrome, were adapted by us to suit the specific problems of writer's cramp (appendix A). For this reason, we psychometrically assessed the reliability of both multi‐item scales in terms of homogeneity.
The WCRS is an objective impairment scale and evaluates the dystonic movements or postures, their latency and duration, occurrence of writing tremor (part A) and writing speed (part B). We used only part A because writing speed was scored as a separate outcome measure (score range 0–16; higher scores reflect more impairment).28
The writing speed was measured as the number of lines of a standard text written within 2 min.
The VAS, SSS and FSS were completed by the patients, whereas the other rating scales were performed by the investigator (JJMK), who was not involved in the injections. Scores after treatment at week 8 were compared with baseline scores.
We enquired about adverse events (pain, weakness, flu‐like symptoms and any other negative effect) on each visit. Weakness was measured objectively using the MRC scale.
Statistical analysis
To identify an appropriate sample size, we based our power calculations on the assumption that BoNT‐A treatment will give a favourable outcome in 50% of patients, and placebo treatment in 10% of patients. With these assumptions, recruitment of 40 patients (20 in each group) provided 80% power to detect a significant difference at the 5% level (two sided).
The homogeneity (or statistical coherence of the scale items) of the SSS and FSS at baseline and 8 weeks follow‐up was expressed in terms of Cronbach's α coefficient and mean item‐total correlation.
The baseline characteristics of the patients were summarised using descriptive statistics. The effect size of the primary treatment outcome was expressed in terms of absolute risk difference. The difference in percentages between the study groups was assessed using the χ2 statistic (or Fisher's exact test if appropriate). Also, we adjusted the primary outcome estimate for the baseline covariates (sex, age, duration of illness, type of writer's cramp), using multivariate logistic regression (effect size expressed as odds ratio (OR)). Logistic modelling was also used to test for the possible presence of interaction between treatment effects and type of writer's cramp (simple v complex).
With regard to the secondary outcomes, we calculated, per group, the mean change in scores from baseline to follow‐up at 8 weeks. The mean change in scores of the patients receiving BoNT‐A and those receiving the control drug were compared using the unpaired t test.
Finally, the follow‐up scores of the rating scales were analysed using multiple linear regression, taking into account the baseline values of the concerning scales, as well as patient's sex, age, duration of illness and type of writer's cramp.
Statistical uncertainty was expressed in 95% confidence limits (CL).
Analyses were done according to the intention‐to‐treat principle and undertaken in accordance with an analysis plan agreed by the trial steering committee before unblinding. All analyses were performed using SPSS V.11.5 software.
Results
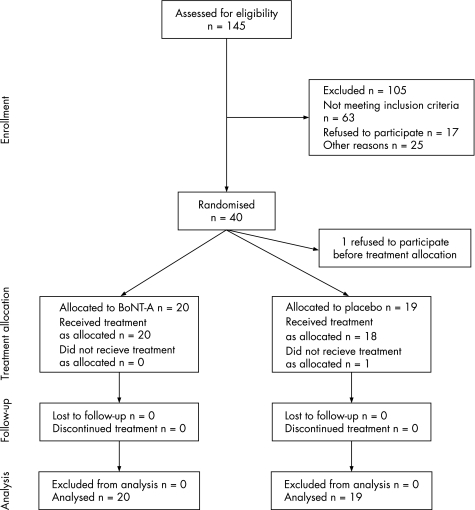
Figure 1 shows the trial flow diagram. Forty patients were enrolled in the trial, 20 in each treatment group. One patient withdrew from the trial in favour of an alternative treatment, before the baseline assessments and treatment; therefore, he was not included in the analysis. Of the remaining 39 patients, 20 in the BoNT‐A treatment group and 19 in the placebo group completed the trial. One patient in the placebo group did not receive treatment as allocated at the second treatment session. Owing to a mistake at the central trial pharmacy, the second trial injections contained BoNT‐A instead of placebo. According to the intention‐to‐treat principle, his data were analysed in the placebo group.
Figure 1 Trail flow diagram.
Table 1 shows the baseline demographic and clinical characteristics according to assigned treatment. In general, patients' demographics were well matched, although some imbalance might be observed with regard to sex, disease duration and functional status. There were no patients lost to follow‐up; all continued treatment and completed assessments until the end of the trial.
Table 1 Baseline demographic and clinical characteristics of the enrolled patients (n = 39).
| Variable | Placebo (SD) | BoNT‐A (SD) |
|---|---|---|
| No of patients | 19 | 20 |
| Women (no of patients) | 7 | 10 |
| Mean age (years) | 45.63 (7.90) | 47.60 (11.24) |
| Mean duration of illness (years) | 9.13 (9.90) | 7.38 (6.22) |
| Simple/complex (no of patients) | 6/13 | 7/13 |
| VAS handwriting | 1.78 (1.39) | 1.70 (1.20) |
| Symptom severity scale (points) | 27.32 (4.57) | 28.10 (4.67) |
| Functional status scale (points) | 8.95 (5.41) | 10.25 (7.02) |
| Writer's cramp rating scale (points) | 4.47 (1.84) | 4.50 (1.96) |
| Writing speed (lines/2 min) | 7.93 (3.20) | 7.59 (2.80) |
BoNT‐A, botulinum toxin type A; VAS, visual analogue scale.
Twenty patients received BoNT‐A treatment. The mean total dose of BoNT‐A (Dysport) in these 20 patients was 102 mouse units (range 30–220) at the first treatment session and 75 at the second session (range 0–240). The mean total dose for both sessions was 178 mouse units. The musculus flexor pollicis longus was the most commonly injected muscle, followed by the musculus flexor digitorum profundus ramus digiti 2 and musculus extensor indices proprius.
Nineteen patients received placebo treatment. The mean total dose of placebo was equal in volume to 82 mouse units (range 20–120) at the first treatment session and 142 at the second (range 0–280). The mean total dose for both sessions was 224 mouse units. The most commonly injected muscles were the same as in the BoNT‐A group.
In the BoNT‐A group, in seven patients no injections were administered at the second session, because they were satisfied with the improvement after the first session. In five patients a double dose was given at the second session because there was no or minimal response, and in eight patients the injection site or dose was altered because of a partial response. In the placebo group, three patients received no injection at the second session, because they were satisfied with the improvement. In 13 patients the dose was doubled because they had no response, and in three patients the injection site or dose was adjusted.
Treatment outcomes
In the BoNT‐A group 14 of 20 patients (70%) wished to continue treatment versus 6 of 19 patients (31.6%) in the placebo group: absolute risk difference = 38.4% (95% CL = 9.4%/67.4%, Fisher's exact test: p = 0.03) in favour of the BoNT‐A group. Logistic regression, adjusting for the baseline covariates, also showed a substantial and significant treatment effect (OR = 9.9; 95% CL = 1.6/59.4, p = 0.01). We could not find evidence any treatment difference between the subgroups of patients with simple or complex writer's cramp (interaction test: p = 0.16).
Six patients in the BoNT‐A group did not wish to continue their treatment: one patient experienced no improvement or weakness at all after two treatment sessions; two patients did have a good subjective and objective improvement on writing but did not wish to continue treatment because of weakness, and one of them also because of pain at the injection site; two had mild subjective improvement, but accompanied by an unacceptable weakness. Finally, one patient had weakness without improvement.
The patient in the placebo group who received placebo at the first treatment session but BoNT‐A at the second treatment session experienced no improvement after the first, but a good improvement after the second treatment.
Psychometric analyses indicated sufficient homogeneity of the SSS and FSS scores at baseline and 8‐week follow up. The range of Cronbach's α coefficients and mean item‐total correlations (r) of the SSS was 0.76–0.79 and r = 0.24–0.28, respectively. The α coefficients of the FSS at both assessments were 0.86, with mean item‐total correlations ranging from 0.32 to 0.34.
Table 2 summarises the secondary outcome scores. Significant improvement was observed with regard to: VAS handwriting, SSS, WCRS and writing speed. No significant difference in change scores could be shown with regard to the FSS.
Table 2 Secondary treatment outcomes according to assigned treatment.
| Secondary outcomes | Placebo (n = 19) | BoNT‐A (n = 20) | Mean difference (95% CL) | p Value* | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 8 weeks | Change | Baseline | 8 weeks | Change | |||
| VAS handwriting (points) | 1.78 (1.39) | 2.31 (1.49) | 0.53 (1.51) | 1.70 (1.20) | 3.55 (1.93) | 1.85 (1.54) | 1.32 (0.33/2.31) | 0.01 |
| Symptom severity scale (points) | 27.32 (4.57) | 26.16 (4.30) | −1.16 (3.18) | 28.10 (4.67) | 24.50 (6.02) | −3.60 (3.20) | −2.44 (−4.52/−0.37) | 0.02 |
| Functional status scale (points) | 8.95 (5.41) | 7.53 (3.95) | −1.42 (1.87) | 10.25 (7.02) | 10.90 (7.93) | 0.65 (5.00) | 2.07 (−0.40/4.54) | 0.10 |
| Writer's cramp rating scale (points) | 4.47 (1.84) | 3.68 (1.16) | −0.79 (1.40) | 4.50 (1.96) | 2.20 (1.88) | −2.30 (1.78) | −1.51 (−2.55/−0.47) | <0.01 |
| Writing speed (lines) | 7.93 (3.20) | 8.20 (2.90) | 0.27 (1.33) | 7.59 (2.80) | 9.00 (2.38) | 1.41 (1.91) | 1.14 (0.07/2.20) | 0.04 |
BoNT‐A, botulinum toxin type A; VAS, visual analogue scale.
Values are mean (SD).
*Unpaired t test.
Adjusted analyses were in close agreement with the unadjusted comparisons. Multiple linear regression analyses (with the follow‐up rating scores as the dependent variables), adjusting for the baseline covariates, including the relevant baseline values of the rating scales, showed significant treatment effects on VAS handwriting (p = 0.02), the WCRS (p<0.01) and writing speed (p = 0.04). The difference between the adjusted SSS follow‐up scores was borderline significant (p = 0.06), whereas no significant beneficial treatment effect was observed regarding the FSS (p = 0.14).
Adverse events
Adverse effects were systematically asked about and examined at each visit. There were only two types of adverse events reported as treatment‐related: weakness in the hand and pain at the injection site. Both were always temporary and recovered completely.
Weakness in the hand was reported by 18 patients in the BoNT‐A group and by two patients in the placebo group. Weakness was observed in 15 patients in the BoNT‐A group and in one patient in the placebo group. The paresis was restricted to the muscles that were injected and varied from 3 to 5‐ on the MRC scale. Pain at the injection site was reported by three patients in the placebo group and by one patient after BoNT‐A injection and lasted at most for 1 week.
One‐year post‐trial follow‐up
After completion of the randomised controlled trial, all 19 patients of the placebo arm were offered BoNT‐A treatment. One patient who had a positive effect on placebo refrained from BoNT‐A. One patient who responded to placebo had a partial response to BoNT‐A, but stopped the treatment because he retired. Three patients who responded to placebo had a good response to BoNT‐A and continued the treatment. Of the 13 patients who did not respond to placebo, nine had a positive response to BoNT‐A. The one patient who received a BoNT‐A injection by mistake at the second treatment session did not respond to the first (placebo) injection, but had a good response to BoNT‐A. In summary, 13 of 18 patients (72%) of the placebo arm, had a positive response to BoNT‐A.
All 20 patients initially treated with BoNT‐A in the trial were offered follow‐up treatment with BoNT‐A. Of the six patients who did not respond to BoNT‐A, five refrained from further BoNT‐A treatment and one had a positive effect after four sessions. Of the 14 patients with a positive response during the trial, six patients stopped in the course of the year after the end of the randomised trial. Four of these patients considered the effect after repeated sessions insufficient, one patient found the travelling distance too long, and one patient retired, and, therefore, his writer's cramp was no longer a problem. Eight patients were still under treatment with a positive effect after 1 year. One patient had a long‐lasting response after two treatment sessions.
After 1 year, 20 of 39 patients (51%) were still under treatment with positive results.
A positive effect after one or more treatment sessions was achieved in 28 of 38 patients (74%). The mean effect duration was 4.7 months (range 3–18). The mean dose per session was 100 IU (range 15–240). Usually two muscles were injected (range 1–4). During the follow‐up period, in seven patients the same muscles were injected, in two patients the dosage was changed, in seven patients fewer muscles were injected, in two patients more muscles were injected and in one patient different muscles were injected.
The occurrence of weakness was not always related to higher dosages of BoNT‐A.
Reasons to stop were insufficient effect, loss of effect, side effects (predominantly weakness), travel distance and retirement.
Discussion
The overall treatment response in our study was significantly better in the BoNT‐A group than in the placebo group, as shown by various objective and subjective levels of measurement. However, not all outcome measures reflect this improvement. The FSS showed no significant difference in the change scores between the two treatment arms. This can be explained by a deterioration of several item scores owing to BoNT‐A‐induced weakness, although the scores for the writing items improved. A limitation of the FSS is that it does not distinguish the disability of dystonia from that of paresis.
A problem in randomised, placebo‐controlled studies on the effect of BoNT‐A treatment is the unblinding of the patient as a result of weakness. This problem is difficult to deal with, as the BoNT‐A‐induced improvement in the dystonia is often accompanied by weakness. To deal with this problem, the treatment effect was also measured with several objective‐rating scales, obtained by a blinded investigator. We found that weakness was not confined to the BoNT‐A group, as two patients in the placebo group also reported weakness after injection.
The results of open‐label studies on the treatment of writer's cramp with BoNT‐A have been encouraging, reporting favourable effects in 60–90% of patients.29,30,31 Three randomised, double‐blind, placebo‐controlled studies have been undertaken, however, with small numbers of patients, different methods and inconclusive results.23,24,25 One study included 20 patients, who were treated once with fixed doses of BoNT‐A.23 In this study only four patients had a subjective improvement in writing, whereas objective assessment of writing improved significantly as a group after BoNT‐A treatment. As each patient received only one active treatment, the observed effects may not have been optimal. The second trial included nine patients with writer's cramp: one dropped out, eight completed the trial.24 Objective evaluation by videotape and handwriting analysis failed to show any difference between toxin and placebo. This was ascribed to inadequate means of evaluation. In the third double‐blind trial, six patients with writer's cramp were included; all had already benefited from BoNT‐A before.25 Three of six improved on subjective and objective testing. It is not possible to compare these trials with each other, because the assessments and the selection criteria used in these trials were different. Furthermore, no attention was paid to the effect of BoNT‐A‐induced weakness on the patients' functioning and their overall opinion of the treatment effect.
Appropriate rating scales are crucial in the evaluation of treatment response. Unfortunately, there are no validated rating scales for focal dystonias. Burke et al developed a quantitative assessment for generalised dystonia that has proved to be valid and statistically reliable,32 but it is insensitive to changes in focal dystonia. Additional scales for focal dystonias were developed by Fahn, which are more specific and more sensitive to slight changes, but the validity and reliability of these rating scales have not yet been determined. We used the FSS and SSS and changed several items to tailor them to writer's cramp; assessment of the statistical reliability of both multi‐item scales in terms of homogeneity was satisfactory.
Most of the studies on the efficacy of BoNT‐A treatment used impairment scales, which measure the direct manifestations of the disease and should be objective, reproducible and sensitive to treatment interventions. However, studies on the relationships between impairment and patients' functioning after treatment with BoNT‐A show inconsistent results.33 Several authors reported differences between the opinions of patients and investigators about the response to treatment.24,25 The specific relationships between impairment and the patient's functional health are, therefore, not clear. Functional health and well‐being are influenced not only by treatment intervention but also by personal and environmental factors—for example, coping capacities, medical attention and social support.33,34,35
Disabilities, the performance of the patient in daily activities, and handicaps, the social disadvantages resulting from impairments and disabilities, are less directly related to the disease, but are closer to the patient's subjective perception of health and well‐being. Our primary outcome measure is a reflection of the patient's subjective perception of health and well‐being, and yet is in close relationship to the direct manifestations of the disease.
Some patients expressed dissatisfaction with treatment, despite substantial improvement in the dystonia. The overall outcome did not meet their expectations or needs, and they felt that the inconvenience of repeated injections or the weakness in the hand outweighed the benefits of treatment. On the other hand, some patients with minimal or mild objective improvement in their dystonia considered the injections very helpful and wished to continue treatment. Continuation of BoNT‐A treatment therefore depends on individual preference, in addition to the degree of achieved improvement in the dystonia.
Our follow‐up data show that about half of the patients were continuing the botulinum treatment with a positive effect after 1 year. This is in line with the two earlier follow‐up reports.29,36
In the follow‐up, the results may be enriched by the exclusion of dropouts, leaving only those patients favourably responding to BoNT‐A treatment. It usually takes several treatment sessions to find the optimal target muscles and BoNT‐A doses for an individual patient. Hence, the treatment can be refined by repeated sessions, leading to more satisfaction.
Writer's cramp remains difficult to treat, but our results show that BoNT‐A injections are safe and effective compared with placebo. After 1 year, about 50% of our patients were still satisfied with BoNT‐A treatment. The role of physical and supportive treatments is unclear. Further investigation of combination treatment seems a promising and challenging task.
Acknowledgements
We thank the patients for their participation in the trial.
Abbreviations
BoNT‐A - botulinum toxin type A
FSS - functional status scale
SSS - symptom severity scale
VAS - visual analogue scale
WCRS - writer's cramp rating scale
Appendix A
Symptom severity scale
The following questions refer to your symptoms for a typical day during the past 2 weeks. Please circle one answer to each question.
-
How long can you write without complaints?
>30 min.
20–30 min.
10–20 min.
1–10 min.
<1 min.
-
How severe is the cramp/spasm of your fingers/wrist/arm during writing?
I have no cramp/spasm.
I have mild cramp/spasm.
I have moderate cramp/spasm, but I can still go on writing.
I have serious cramp/spasm and great difficulty writing.
I cannot write anymore due to extreme cramp/spasm.
-
How severe is your pain during writing?
I do not have pain.
I have mild pain.
I have moderate pain.
I have severe pain.
-
How often do you have to stop writing due to pain or cramp/spasm in your hand/arm?
Never.
Sometimes.
Often.
Very often.
I cannot write anymore.
-
How long does your pain last after writing?
<1 min.
1–10 min.
10–30 min.
>30 min.
-
Do you have weakness of your hand, wrist or arm?
I do not have weakness.
I have mild weakness.
I have moderate weakness.
I have severe weakness.
-
Do you have numbness (loss of sensation) in your hand?
No.
I have mild numbness.
I have moderate numbness.
I have severe numbness.
-
Do your complaints have effect on your daily functioning?
No.
Mild.
Moderate.
Severe.
-
Do your complaints have effect on your occupation?
No.
Yes, but not serious (eg I can use a computer instead of writing).
Yes, serious effect.
I can no longer carry on my occupation, or I've had to change my occupation due to my complaints.
-
Do you write less often due to your complaints?
No.
Yes, 25–50% less.
Yes, 50–75% less.
I hardly ever write anymore.
Functional status scale
On a typical day during the past 2 weeks, have hand/wrist/arm symptoms caused you any difficulty doing the activities listed below? Please circle one number that best describes your ability to do the activity.
Table AI Functional status scale.
| Activity | No difficulty | Mild difficulty | Moderate difficulty | Severe symptoms |
|---|---|---|---|---|
| Writing | 0 | 1 | 2 | 3 |
| Place signature | 0 | 1 | 2 | 3 |
| Typewriting | 0 | 1 | 2 | 3 |
| Buttoning of clothes | 0 | 1 | 2 | 3 |
| Brushing teeth | 0 | 1 | 2 | 3 |
| Applying make‐up or handling a screwdriver | 0 | 1 | 2 | 3 |
| Handling cutlery | 0 | 1 | 2 | 3 |
| Opening a bottle or jar | 0 | 1 | 2 | 3 |
| Holding a book while reading | 0 | 1 | 2 | 3 |
| Carrying grocery bag | 0 | 1 | 2 | 3 |
| Household chores | 0 | 1 | 2 | 3 |
| Personal hygiene and dressing | 0 | 1 | 2 | 3 |
Footnotes
Funding: Trial medication and an unrestricted research grant for the workgroup Movement Disorders at the Academic Medical Centre, University of Amsterdam was received from Ipsen Pharmaceutical. Ipsen Pharmaceutical had no role in study design, collection, analysis, interpretation of data, in the writing of the report and in the decision to submit the paper for publication. The study was funded by a grant from the Prinses Beatrix Fonds (MAR 00‐0119). JHTMK received a travel grant from Allergan. JJMK received a travel grant from Ipsen.
Competing interests: None.
References
- 1.Sheehy M P, Marsden C D. Writers' cramp‐a focal dystonia. Brain 1982105(Pt 3)461–480. [DOI] [PubMed] [Google Scholar]
- 2.Sheehy M P, Rothwell J C, Marsden C D. Writer's cramp. Adv Neurol 198850457–472. [PubMed] [Google Scholar]
- 3.Thompson P D. Writers' cramp. Br J Hosp Med 19935091–94. [PubMed] [Google Scholar]
- 4.Jankovic J, Schwartz K S. Use of botulinum toxin in the treatment of hand dystonia. J Hand Surg 199318883–887. [DOI] [PubMed] [Google Scholar]
- 5.Jedynak P C, Tranchant C, de Beyl D Z. Prospective clinical study of writer's cramp. Mov Disord 200116494–499. [DOI] [PubMed] [Google Scholar]
- 6.The Epidemiological Study of Dystonia in Europe (ESDE) Collaborative Group A prevalence study of primary dystonia in eight European countries. J Neurol 2000247787–792. [DOI] [PubMed] [Google Scholar]
- 7.Butler A G, Duffey P O, Hawthorne M R.et al An epidemiologic survey of dystonia within the entire population of northeast England over the past nine years. Adv Neurol 20049495–99. [PubMed] [Google Scholar]
- 8.Nutt J G, Muenter M D, Aronson A.et al Epidemiology of focal and generalized dystonia in Rochester, Minnesota. Mov Disord 19883188–194. [DOI] [PubMed] [Google Scholar]
- 9.Lees A J, Kleedorfer B, Foster H. Treatment of writers' dystonia. Lancet 198921525. [DOI] [PubMed] [Google Scholar]
- 10.Ranawaya R, Lang A. Usefulness of a writing device in writer's cramp. Neurology 1991411136–1138. [DOI] [PubMed] [Google Scholar]
- 11.Ince L P, Leon M S, Christidis D. EMG biofeedback for handwriting disabilities: a critical examination of the literature. J Behav Ther Exp Psychiatry 19861795–100. [DOI] [PubMed] [Google Scholar]
- 12.Wieck A, Harrington R, Marks I.et al Writer's cramp: a controlled trial of habit reversal treatment. Br J Psychiatry 1988153111–115. [DOI] [PubMed] [Google Scholar]
- 13.Marsden C D, Sheehy M P. Writer's cramp. Trends Neurosci 199013148–153. [DOI] [PubMed] [Google Scholar]
- 14.Jankovic J. Dystonia: medical therapy and botulinum toxin. Adv Neurol 200494275–286. [PubMed] [Google Scholar]
- 15.Candia V, Elbert T, Altenmuller E.et al Constraint‐induced movement therapy for focal hand dystonia in musicians. Lancet 199935342. [DOI] [PubMed] [Google Scholar]
- 16.Priori A, Pesenti A, Cappellari A.et al Limb immobilization for the treatment of focal occupational dystonia. Neurology 200157405–409. [DOI] [PubMed] [Google Scholar]
- 17.Tas N, Karatas G K, Sepici V. Hand orthosis as a writing aid in writer's cramp. Mov Disord 2001161185–1189. [DOI] [PubMed] [Google Scholar]
- 18.Iacono R P, Kuniyoshi S M, Schoonenberg T. Experience with stereotactics for dystonia: case examples. Adv Neurol 199878221–226. [PubMed] [Google Scholar]
- 19.Goto S, Tsuiki H, Soyama N.et al Stereotactic selective Vo‐complex thalamotomy in a patient with dystonic writer's cramp. Neurology 1997491173–1174. [DOI] [PubMed] [Google Scholar]
- 20.Jankovic J, Schwartz K, Donovan D T. Botulinum toxin treatment of cranial‐cervical dystonia, spasmodic dysphonia, other focal dystonias and hemifacial spasm. J Neurol Neurosurg Psychiatry 199053633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brans J W, Lindeboom R, Snoek J W.et al Botulinum toxin versus trihexyphenidyl in cervical dystonia: a prospective, randomized, double‐blind controlled trial. Neurology 1996461066–1072. [DOI] [PubMed] [Google Scholar]
- 22.Sheean G L, Murray N M, Marsden C D. Pain and remote weakness in limbs injected with botulinum toxin A for writer's cramp. Lancet 1995346154–156. [DOI] [PubMed] [Google Scholar]
- 23.Tsui J K, Bhatt M, Calne S.et al Botulinum toxin in the treatment of writer's cramp: a double‐blind study. Neurology 199343183–185. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimura D M, Aminoff M J, Olney R K. Botulinum toxin therapy for limb dystonias. Neurology 199242627–630. [DOI] [PubMed] [Google Scholar]
- 25.Cole R, Hallett M, Cohen L G. Double‐blind trial of botulinum toxin for treatment of focal hand dystonia. Mov Disord 199510466–471. [DOI] [PubMed] [Google Scholar]
- 26.Brans J W, de Boer I P, Aramideh M.et al Botulinum toxin in cervical dystonia: low dosage with electromyographic guidance. J Neurol 1995242529–534. [DOI] [PubMed] [Google Scholar]
- 27.Atroshi I, Breidenbach W C, McCabe S J. Assessment of the carpal tunnel outcome instrument in patients with nerve‐compression symptoms. J Hand Surg 199722222–227. [DOI] [PubMed] [Google Scholar]
- 28.Wissel J, Kabus C, Wenzel R.et al Botulinum toxin in writer's cramp: objective response evaluation in 31 patients. J Neurol Neurosurg Psychiatry 199661172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karp B I, Cole R A, Cohen L G.et al Long‐term botulinum toxin treatment of focal hand dystonia. Neurology 19944470–76. [DOI] [PubMed] [Google Scholar]
- 30.Rivest J, Lees A J, Marsden C D. Writer's cramp: treatment with botulinum toxin injections. Mov Disord 1991655–59. [DOI] [PubMed] [Google Scholar]
- 31.Koelman J H, Struys M A, Ongerboer de Visseret al Writer's cramp treated with botulinum injections. Ned Tijdschr Geneeskd 19981421768–1771. [PubMed] [Google Scholar]
- 32.Burke R E, Fahn S, Marsden C D.et al Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 19853573–77. [DOI] [PubMed] [Google Scholar]
- 33.Lindeboom R, Brans J W, Aramideh M.et al Treatment of cervical dystonia: a comparison of measures for outcome assessment. Mov Disord 199813706–712. [DOI] [PubMed] [Google Scholar]
- 34.Tennant A. Quality of life‐‐a measure too far? Ann Rheum Dis 199554439–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben‐Shlomo Y, Camfield L, Warner T. What are the determinants of quality of life in people with cervical dystonia? J Neurol Neurosurg Psychiatry 200272608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marion M H, Afors K, Sheehy M P. Problems of treating writer's cramp with botulinum toxin injections: results from 10 years of experience. Rev Neurol 2003159923–927. [PubMed] [Google Scholar]