Abstract
Background
Structural neuroimaging studies have consistently shown a pattern of extra‐hippocampal atrophy in patients with left and right drug‐refractory medial temporal lobe epilepsy (MTLE). However, it is not yet completely understood how extra‐hippocampal atrophy is related to hippocampal atrophy. Moreover, patients with left MTLE often exhibit more intense cognitive impairment, and subtle brain asymmetries have been reported in patients with left MTLE versus right MTLE but have not been explored in a controlled study.
Objectives
To investigate the association between extra‐hippocampal and hippocampal atrophy in patients with MTLE, and the effect of side of hippocampal atrophy on extra‐hippocampal atrophy.
Methods
Voxel‐based morphometry analyses of magnetic resonance images of the brain were performed to determine the correlation between regional extra‐hippocampal grey matter volume and hippocampal grey matter volume. The results from 36 patients with right and left MTLE were compared, and results from the two groups were compared with those from 49 healthy controls.
Results
Compared with controls, patients with MTLE showed a more intense correlation between hippocampal grey matter volume and regional grey matter volume in locations such as the contralateral hippocampus, bilateral parahippocampal gyri and frontal and parietal areas. Compared with right MTLE, patients with left MTLE exhibited a wider area of atrophy related to hippocampal grey matter loss, encompassing both the contralateral and ipsilateral hemispheres, particularly affecting the contralateral hippocampus.
Conclusions
Our results suggest that left hippocampal atrophy is associated with a larger degree of extra‐hippocampal atrophy. This may help to explain the more intense cognitive impairment usually observed in these patients.
Hippocampal sclerosis is the most common underlying condition of drug‐refractory medial temporal lobe epilepsy (MTLE).1 Signs associated with hippocampal sclerosis, such as hippocampal atrophy and increased T2 signal, are reliably detected in vivo by magnetic resonance imaging (MRI).2 Recently, volumetric MRI analyses such as manual morphometry and voxel‐based morphometry (VBM) have shown that atrophy in patients with MTLE involves not only the sclerotic hippocampus but also the surrounding extra‐hippocampal and extra‐temporal structures. Considerable atrophy has been shown in regions such as the ipsilateral medial temporal lobe,3 the thalamus,4,5,6 the cerebellum7,8 and the cingulate cortex.8
Even though the pattern of extra‐hippocampal atrophy is apparently similar in the ipsilateral and contralateral hemispheres in patients with left and right MTLE,8 it is clinically suspected that patients with left MTLE may exhibit a more intense and pervasive atrophy. For example, during preoperative neuropsychological evaluations, patients with left MTLE often exhibit more profound cognitive impairment than patients with right MTLE. Patients with left MTLE show a markedly poorer performance in tasks comprising verbal memory, general memory and delayed recall.9 Nonetheless, deficits in patients with left MTLE are possibly more salient than in patients with right MTLE, because cognitive assessments are more focused on language tests. Moreover, visuospatial deficits can be compensated by language strategies, therefore masking the real intensity of deficits in patients with right MTLE. It is therefore unclear whether neuronal damage is more intense and widespread in patients with left MTLE or whether left and right MTLE are essentially the same condition, mirrored side by side. It is also unclear whether the presence of hippocampal sclerosis in the language‐dominant hemisphere is associated with a different pattern of brain damage and extra‐hippocampal grey matter loss.
Few studies have specifically discussed this question. For example, whole‐brain morphometry studies using VBM have shown that the grey matter loss is different in patients with right MTLE and left MTLE, when these groups are compared with controls.4,5,6,7,8 However, it is not clear whether this is an indication of different pathology, or an effect of statistical power. For instance, variations in the homogeneity of data or sample size in some studies4,6,7,8 could account for the observed differences.
In this study, we aimed to investigate whether left and right MTLE are significantly different with regard to the pattern and intensity of extra‐hippocampal atrophy. We performed a cross‐sectional study investigating the statistical differences between left and right MTLE grey matter volume atrophy. It is currently difficult to predict whether left and right MTLE are associated with different patterns of resultant atrophy, or whether the progression of regional atrophy is different between the two groups. Therefore, we aimed to evaluate, by using a cross‐sectional design, the correlation of extra‐hippocampal grey matter reduction associated with hippocampal atrophy. Using this approach, we investigated whether the variation in regional grey matter is dependent on hippocampal atrophy, and whether this pattern is different in the two groups. The effect of duration of epilepsy was also explored. We hypothesised that patients with left MTLE would exhibit a more profound and widespread extra‐hippocampal atrophy, in association with hippocampal atrophy.
Methods
Participants
In all, 36 consecutive patients with drug‐refractory MTLE were enrolled in this study (13 men, mean (standard deviation (SD)) age 35 (8.9) years, range 17–54 years). Patients were referred from the epilepsy clinic of the State University of Campinas, Campinas, São Paulo, Brazil, where they were diagnosed on the basis of comprehensive neurological evaluation, including medical history, neurological examination, interictal electroencephalogram (EEG) and prolonged video‐EEG monitoring. Diagnosis of MTLE was based on International League Against Epilepsy criteria,10 and seizures were clinically lateralised according to the combination of the data from the neurological examination, interictal and prolonged EEG with recording of onset of seizure. Data from clinical and electrophysiological evaluations were concordant for all patients, all exhibited only unilateral onset of seizures. Patients also underwent routine MRI, which further confirmed the laterality of MTLE: all patients presented unilateral hippocampal atrophy, ipsilateral to the side of origin of seizure, confirmed by senior neurologists and neuroradiologists. Only consecutive patients with a homogeneous clinical profile—that is, drug‐refractory MTLE due to unilateral hippocampal sclerosis, with concordant unilateral onset of seizures—were included in this study. In all, 19 patients had left MTLE and 17 had right MTLE.
Patients were evaluated with regard to hemispheric dominance by the Edinburgh Handedness Inventory11 combined with the Auditory Discrimination and Dichotic Listening Tests.12 A total of 18 of the 19 patients with left MTLE, and 16 of 17 patients with right MTLE were right handed. The left‐handed patient with left MTLE exhibited a left hemispheric dominance for language. The left‐handed patient with right MTLE exhibited a bilateral hemispheric dominance for language. Taken together, our sample was constituted by a great majority of, if not all, patients with left hemispheric dominance.
The duration of epilepsy was determined by interviewing the patient and at least one relative who lived in close contact with the patient at the time when the seizures started. The time of onset of epilepsy was estimated from the date of the initial complex partial seizures. The time of symptoms ranged from 10 to 49 years (mean (SD) 28.7 (9.3) years). All patients signed a written informed consent approved by the institutional review board of the State University of Campinas, Brazil, where the MRI scanning was performed. We also acquired MRI scans from 49 neurologically healthy people (18 men, mean (SD) age 31 (8) years, range 20–60 years). We found no significant difference in age (F2, 84 = 2.3; p = 0.11) or sex (Yates' χ2 df1 = 0.03; p = 0.86) distribution between controls and patients with MTLE.
Neuroimaging: data preprocessing
All participants underwent magnetic resonance scanning on a 2T scanner (Elscint Prestige, Haifa, Israel). T1‐weighted images (TR = 22 ms, TE = 9 ms, flip angle = 35°, matrix = 256×220, field of view = 25×22 cm, sagittal acquisition) with either 1 mm isotropic voxels or 1.5×0.97×0.97 mm. Raw DICOM format images were transformed into Analyze format using MRIcro software (www.mricro.com)13. Images were preprocessed using built‐in routines of the software package SPM2 (http://www.fil.ion.ucl.ac.uk/spm), according to an “optimised” VBM protocol. Images were normalised, aiming to transfer the size and shape of each individual brain to the standard stereotaxic space. Normalisation was performed using 12 linear and 7×8×7 non‐linear basis functions; in addition, a brain mask was used to ensure that the fit was based on the shape of the brain rather than on the surrounding scalp. Spatially normalised images were resliced to an isotropic 1.5 mm voxel size, and subsequently submitted to tissue segmentation using SPM2's built‐in routines for “optimised VBM”, which estimate the probability of a grey matter density for each voxel. To overcome artificial distortion of brain structure during normalisation, we modulated the estimated concentration of tissue in segmented images based on the spatial deformations selected during normalisation,14 preserving the quantity of grey matter while ensuring a good spatial alignment. Finally, normalised grey matter segmented maps were convolved with a 10‐mm isotropic Gaussian kernel filter to minimise interindividual variability of sulci and gyri, and to minimise false‐positive findings.15 This smoothing creates images that are more normally distributed and allows voxelwise analysis.
Neuroimaging: statistical analyses
Statistical analyses were performed on normalised, modulated and smoothed probabilistic maps of segmented grey matter. Different forms of statistical analyses were performed, using general linear model paradigms implemented into SPM2. All analyses were performed estimating the probability of the major effects voxel by voxel, followed by correction of the statistical threshold for the effect of multiple comparisons. A 5% false discovery rate statistical threshold was adopted to control for multiple comparisons.16 In the context of VBM, a false discovery rate of 0.05 implies that approximately 5 of 100 voxels identified as significant are actually false alarms.
Statistical analyses consisted of the following.
1. Evaluation of areas in the brain network where grey matter volumes correspond to hippocampal grey matter. The density of hippocampal grey matter was extracted from the hippocampus ipsilateral to the focus of seizure in patients with MTLE. The hippocampal region of interest was set, unmodified, according to the Anatomical Automatic Labeling Freeware (http://www.cyceron.fr/freeware/). The grey matter intensity from the hippocampal region of interest was extracted using the software package Marsbar,17 exported, and then used as a covariate for every patient in a whole‐brain voxelwise simple regression.
2. Comparison of hippocampal connected networks between patients and controls. The investigation of areas where grey matter is correlated with hippocampal grey matter can suggest a network of synchronous atrophy in MTLE.18 However, the correlation between grey matter and hippocampal grey matter in regions of the brain can also exist in normal people. Therefore, we examined the regions where grey matter is correlated with hippocampal grey matter in normal participants, using the technique described above for patients with MTLE. Subsequently, we used a multiple regression analysis investigating whether patients with left or right MTLE would exhibit a different pattern of grey matter areas where variation of grey matter is correlated with hippocampal grey matter, suggesting a synchronous atrophy in MTLE. We investigated whether the correlation with hippocampal grey matter was more intense in patients than in controls. The contrasts used investigated correlation in left MTLE > correlation in controls and correlation in right MTLE > correlation in controls.
3. Synchronous atrophy in left MTLE compared with right MTLE. Using multiple regressions with hippocampal grey matter intensity as a predictor, we investigated differences between the patterns of grey matter variation in association with hippocampal atrophy in patients with left MTLE compared with those with right MTLE. The contrast investigated correlation in right MTLE > correlation in controls > correlation in right MTLE > correlation in controls.
4. Effect of duration of epilepsy on synchronous atrophy in left MTLE compared with right MTLE. Differences between the grey matter variation in association with hippocampal atrophy in patients with left and right MTLE were investigated using multiple regression, with the effects of time of symptoms covaried out. The contrast used was similar to the one used to investigate synchronous atrophy in left MTLE compared with right MTLE, with duration of epilepsy used as a covariate for multiple regressions.
All the above analyses were performed using SPM2. We did not use grand mean scaling, but we used proportional threshold masking (set to 0.8) and brain masking.
The stereotaxic coordinates were converted to Talairach coordinates and to anatomical names using the Talairach Daemon client (http://ric.uthscsa.edu/projects/talairachdaemon.html).
Results
Evaluation of areas in the brain network where grey matter volumes correspond to hippocampal grey matter
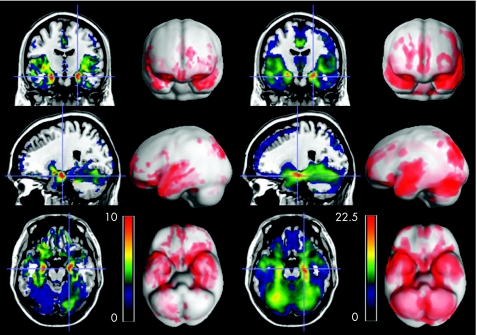
Various structures in the brain of patients with MTLE showed a correlation between regional grey matter and the grey matter density from the atrophied hippocampus. Naturally, the hippocampus ipsilateral to the hippocampal sclerosis was the region with the strongest correlation. Regions such as the contralateral hippocampus, both parahippocampal gyri and insulae, were strongly associated with hippocampal grey matter. In addition, grey matter volume of the temporal isocortex, putamen, uncus, cerebellum, hypothalamus, parietal and frontal lobes were also correlated with hippocampal grey matter density. Table 1 and fig 1 outline these results.
Table 1 Areas where grey matter is correlated with hippocampal grey matter in healthy controls.
| Anatomical location | Spatial coordinates | Voxelwise | ||||
|---|---|---|---|---|---|---|
| Side | Location | x | y | z | T | z |
| Ipsilateral | Hippocampus/amygdala | 24 | −9 | −17 | 11.79 | 6.07 |
| Hippocampus | 29 | −21 | −15 | 10.95 | 5.88 | |
| Parahippocampal gyrus | 24 | 3 | −14 | 10.12 | 5.69 | |
| Middle temporal gyrus | 57 | −21 | −5 | 8.79 | 5.33 | |
| Inferior parietal lobule | 50 | −27 | 23 | 8.25 | 5.17 | |
| Cerebellum | 24 | −36 | −32 | 7.55 | 4.94 | |
| Sub‐gyral | 39 | −9 | −8 | 7.46 | 4.91 | |
| Insula | 44 | −9 | 8 | 7.44 | 4.9 | |
| Lingual gyrus | 15 | −90 | −17 | 7.1 | 4.78 | |
| Lingual gyrus | 12 | −75 | −9 | 6.94 | 4.72 | |
| Insula | 41 | −17 | 8 | 6.9 | 4.7 | |
| Parahippocampal gyrus | 36 | −30 | −21 | 6.88 | 4.69 | |
| Hypothalamus | 6 | −2 | −9 | 6.76 | 4.65 | |
| Middle frontal gyrus | 29 | 28 | 46 | 5.42 | 4.07 | |
| Inferior parietal lobule | 33 | −37 | 54 | 4.56 | 3.63 | |
| Superior parietal lobule | 17 | −63 | 56 | 4.47 | 3.59 | |
| Precentral gyrus | 56 | −15 | 28 | 3.57 | 3.04 | |
| Medial frontal gyrus | 6 | −14 | 63 | 3.43 | 2.95 | |
| Postcentral gyrus | 50 | −24 | 48 | 2.67 | 2.41 | |
| Middle frontal gyrus | 44 | 48 | −7 | 2.64 | 2.38 | |
| Inferior parietal lobule | 55 | −41 | 29 | 2.54 | 2.31 | |
| Contralateral | Hippocampus | −23 | −12 | −21 | 11.06 | 5.91 |
| Subcallosal gyrus | −18 | 3 | −11 | 10.99 | 5.89 | |
| Fusiform gyrus | −41 | −32 | −17 | 8.6 | 5.27 | |
| Insula | −42 | −5 | 9 | 8.58 | 5.27 | |
| Middle temporal gyrus | −50 | −38 | 5 | 8.5 | 5.24 | |
| Insula | −35 | −23 | 6 | 8.44 | 5.22 | |
| Sub‐gyral | −38 | −9 | −5 | 8.06 | 5.11 | |
| Orbital gyrus | −21 | 30 | −21 | 7.82 | 5.03 | |
| Parahippocampal gyrus | −27 | −21 | −17 | 7.7 | 4.99 | |
| Putamen | −27 | −14 | 5 | 7.49 | 4.92 | |
| Inferior temporal gyrus | −53 | −5 | −33 | 7.46 | 4.91 | |
| Insula | −39 | 5 | −5 | 7.33 | 4.86 | |
| Uncus | −23 | −6 | −30 | 7.32 | 4.86 | |
| Insula | −39 | −5 | −3 | 7.25 | 4.83 | |
| Parahippocampal gyrus | −27 | −27 | −23 | 7.12 | 4.78 | |
| Superior temporal gyrus | −30 | 12 | −39 | 6.77 | 4.65 | |
| Superior temporal gyrus | −51 | 0 | 0 | 6.77 | 4.65 | |
| Inferior parietal lobule | −42 | −46 | 52 | 6.28 | 4.46 | |
| Sub‐gyral | −27 | −5 | 56 | 5.51 | 4.12 | |
| Middle frontal gyrus | −38 | 31 | 36 | 4.13 | 3.39 | |
| Postcentral gyrus | −56 | −19 | 29 | 4.04 | 3.34 | |
| Precentral gyrus | −55 | −6 | 28 | 3.87 | 3.23 | |
| Superior frontal gyrus | −21 | 47 | 35 | 3.41 | 2.94 | |
| Inferior parietal lobule | −45 | −55 | 50 | 3.41 | 2.93 | |
| Superior temporal gyrus | −50 | −53 | 19 | 2.85 | 2.54 | |
| Medial frontal gyrus | −5 | −23 | 70 | 2.68 | 2.41 | |
| Superior frontal gyrus | −32 | 22 | 51 | 2.44 | 2.23 | |
FDR, false discovery rate; MTLE, medial temporal lobe epilepsy.
Correlated with the ipsilateral hippocampus in patients with MTLE.
Height threshold: T = 1.98, cluster ⩾20 voxels, FDR corrected (p<0.05).
Figure 1 Regional correlation of grey matter with hippocampal grey matter. The first two columns represent the correlation with atrophied hippocampal grey matter in patients with medial temporal lobe epilepsy. The ipsilateral hemisphere is shown in the right side of the brain (cross‐hair highlighting the hippocampal area). The two columns on the right show the regional correlation in controls. The statistical maps on slices represent z scores, and the exponentially weighted integral of the test statistic is overlaid on a smoothed cortical rendering.
Comparison of hippocampal connected networks between patients and controls
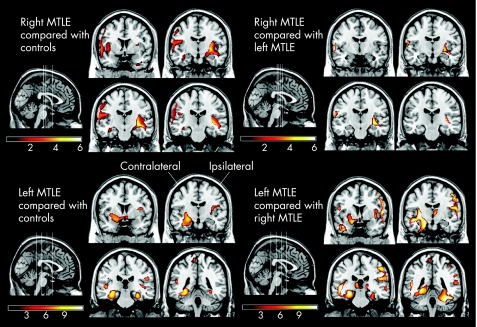
Patients with both right and left MTLE exhibited a pattern of regional grey matter variation correlated with hippocampal grey matter, which was significantly different from the pattern exhibited by controls. More specifically, patients with left MTLE exhibited a significant association in grey matter volume of the contralateral hippocampus, bilateral parahippocampal gyri, bilateral precuneus, ipsilateral insula, bilateral frontal and parietal regions, ipsilateral occipital regions and ipsilateral fusiform gyrus. Also, compared with controls, patients with right MTLE exhibited significant association of grey matter in locations such as the ipsilateral putamen, ipsilateral and contralateral temporal isocortex, bilateral cuneus and precuneus, contralateral hippocampus, bilateral parahippocampal cortex, contralateral caudate, contralateral cingulate, bilateral frontal regions, contralateral parietal regions and ipsilateral occipital regions. Table 2 and fig 2 summarise these results.
Table 2 Areas where grey matter is significantly more associated with hippocampal grey matter in patients with left and right medial temporal lobe epilepsy compared with controls.
| Correlation with ipsilateral hippocampal grey matter more intense in patients with left MTLE, compared with controls | Correlation with ipsilateral hippocampal grey matter more intense in patients with right MTLE, compared with controls | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Height threshold: T = 2.80 | Height threshold: T = 2.74 | ||||||||||||
| Anatomical location | Spatial coordinates | Voxelwise | Anatomical location | Spatial coordinates | Voxelwise | ||||||||
| Side | Location | x | y | z | T | z | Side | Location | x | y | z | T | z |
| Ipsilateral | Parahippocampal gyrus | 30 | −30 | −13 | 5.48 | 5.12 | Ipsilateral | Putamen | 32 | −17 | −1 | 11.95 | Inferior |
| Insula | 38 | 6 | 13 | 4.14 | 3.97 | Superior temporal gyrus | 50 | −23 | −6 | 7.04 | 6.35 | ||
| Inferior parietal lobule | 36 | −50 | 57 | 3.84 | 3.7 | Cuneus | 8 | −85 | 36 | 5.37 | 5.03 | ||
| Inferior frontal gyrus | 21 | 33 | −17 | 3.69 | 3.56 | Middle frontal gyrus | 24 | 28 | −17 | 4.15 | 3.98 | ||
| Middle frontal gyrus | 39 | 46 | 8 | 3.65 | 3.53 | Inferior occipital gyrus | 17 | −93 | −10 | 4.01 | 3.86 | ||
| Postcentral gyrus | 53 | −24 | 23 | 3.52 | 3.41 | Precuneus | 36 | −82 | 34 | 3.51 | 3.4 | ||
| Superior frontal gyrus | 2 | 53 | 30 | 3.48 | 3.38 | Cuneus | 33 | −85 | 31 | 3.48 | 3.38 | ||
| Precuneus | 18 | −73 | 41 | 3.42 | 3.32 | Superior temporal gyrus | 33 | 7 | −32 | 3.43 | 3.33 | ||
| Middle frontal gyrus | 30 | 13 | 52 | 3.39 | 3.3 | Postcentral gyrus | 8 | −48 | 63 | 3.03 | 2.96 | ||
| Middle occipital gyrus | 36 | −80 | 17 | 3.18 | 3.09 | Parahippocampal gyrus | 24 | −13 | −26 | 2.93 | 2.86 | ||
| Middle frontal gyrus | 27 | −3 | 61 | 3.09 | 3.02 | ||||||||
| Inferior temporal gyrus | 50 | −23 | −11 | 3.01 | 2.94 | Contralateral | Middle temporal gyrus | −58 | 5 | −7 | 7.62 | 6.77 | |
| Middle occipital gyrus | 36 | −87 | 0 | 3 | 2.93 | Superior temporal gyrus | −58 | 3 | 2 | 7.13 | 6.42 | ||
| Fusiform gyrus | 42 | −46 | −9 | 2.97 | 2.9 | Precuneus | −11 | −56 | 30 | 5.29 | 4.96 | ||
| Superior frontal gyrus | −38 | 50 | 22 | 5.2 | 4.89 | ||||||||
| Contralateral | Hippocampus | −30 | −32 | −3 | 6.12 | 5.64 | Caudate tail | −30 | −35 | 2 | 4.92 | 4.65 | |
| Parahippocampal gyrus | −30 | −44 | −3 | 5.63 | 5.24 | Parahippocampal gyrus | −27 | −49 | 1 | 4.91 | 4.64 | ||
| Precuneus | −5 | −55 | 64 | 4.53 | 4.32 | Hippocampus | −36 | −17 | −12 | 4.35 | 4.16 | ||
| Middle occipital gyrus | −35 | −81 | 20 | 4 | 3.84 | Fusiform gyrus | −47 | −52 | −7 | 4.87 | 4.61 | ||
| Precuneus | −17 | −73 | 44 | 3.78 | 3.65 | Middle temporal gyrus | −35 | −80 | 21 | 4.59 | 4.36 | ||
| Rectal gyrus | −5 | 45 | −24 | 3.64 | 3.52 | Inferior parietal lobule | −49 | −29 | 46 | 4.33 | 4.14 | ||
| Middle frontal gyrus | −41 | 39 | 15 | 3.49 | 3.39 | Middle frontal gyrus | −27 | 19 | 54 | 3.91 | 3.77 | ||
| Superior frontal gyrus | −30 | 22 | 50 | 3.38 | 3.28 | Middle frontal gyrus | −35 | 3 | 55 | 3.79 | 3.66 | ||
| Medial frontal gyrus | −2 | −19 | 64 | 3.3 | 3.21 | Superior frontal gyrus | −11 | 52 | 19 | 3.71 | 3.59 | ||
| Inferior parietal lobule | −49 | −34 | 28 | 3.24 | 3.16 | Cuneus | −11 | −81 | 4 | 3.67 | 3.55 | ||
| Middle frontal gyrus | −35 | 36 | −16 | 3.2 | 3.12 | Anterior cingulate | −11 | 43 | −10 | 3.16 | 3.08 | ||
| Inferior frontal gyrus | −44 | 21 | 4 | 3.02 | 2.95 | Caudate head | −8 | 5 | −2 | 3.12 | 3.05 | ||
| Cingulate gyrus | 0 | 16 | 34 | 2.93 | 2.87 | ||||||||
FDR, false discovery rate; ; MTLE, medial temporal lobe epilepsy.
FDR corrected (p<0.05), only clusters >10 voxels
Figure 2 Differences in correlation of regional grey matter with atrophied hippocampal grey matter. The statistical maps are voxelwise representations of z scores of areas where the correlation is stronger in patients with right medial temporal lobe epilepsy (MTLE) versus controls (top left quadrant, sagittal representation of slices and corresponding four coronal slices); in patients with left MTLE versus controls (bottom left quadrant), in patients with right MTLE compared with left MTLE (top right quadrant) and in patients with left MTLE compared with right MTLE (bottom right quadrant).
Synchronous atrophy in left MTLE compared with right MTLE
Comparing the contrasts of correlations between patients and controls, patients with left MTLE exhibited a significantly different pattern of regional grey matter loss correlated with hippocampal grey matter, in comparison with those with right MTLE.
Patients with left MTLE exhibited a more significant association with hippocampal grey matter loss in the contralateral hemisphere in the putamen, hippocampus, superior temporal gyrus and inferior frontal gyrus. In the hemisphere ipsilateral to hippocampal atrophy, a more intense correlation was observed in the fusiform gyrus, superior frontal gyrus, inferior parietal lobule, cuneus, middle occipital gyrus, middle frontal gyrus, cingulate gyrus and hypothalamus.
Patients with right MTLE exhibited a more intense correlation in the ipsilateral putamen and parahippocampal gyrus, and in the contralateral middle temporal gyrus, precentral gyrus, superior temporal gyrus, inferior frontal gyrus and supramarginal gyrus. Table 3 and fig 2 summarise these results.
Table 3 Areas where grey matter is significantly more associated with hippocampal grey matter in patients with left medial temporal lobe epilepsy compared with right medial temporal lobe epilepsy.
| Correlation with ipsilateral hippocampal grey matter more intense in patients with left MTLE compared with those with right MTLE | Correlation with ipsilateral hippocampal grey matter more intense in patients with right MTLE compared with those with left MTLE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Height threshold: T = 2.31 | Height threshold: T = 3.51 | ||||||||||||
| Voxelwise | Spatial coordinates | Anatomical location | Voxelwise | Spatial coordinates | Anatomical location | ||||||||
| T | z | x | y | z | Side | Location | T | z | x | y | z | Side | Location |
| 7.31 | 6.55 | 38 | −39 | −21 | Ipsilateral | Fusiform gyrus | 7 | 6.32 | 32 | −17 | −1 | Ipsilateral | Putamen |
| 4.5 | 4.29 | 9 | 47 | 34 | Ipsilateral | Superior frontal gyrus | 5.4 | 5.06 | −58 | 5 | −7 | Contralateral | Middle temporal gyrus |
| 4.15 | 3.98 | 48 | −53 | 39 | Ipsilateral | Inferior parietal lobule | 5.05 | 4.76 | −61 | −5 | 10 | Contralateral | Precentral gyrus |
| 4.01 | 3.86 | 17 | −92 | 3 | Ipsilateral | Cuneus | 4.76 | 4.51 | −58 | 3 | 2 | Contralateral | Superior temporal gyrus |
| 3.46 | 3.36 | 45 | −78 | 4 | Ipsilateral | Middle occipital gyrus | 4.41 | 4.21 | −42 | 14 | −5 | Contralateral | Inferior frontal gyrus |
| 3.39 | 3.29 | 30 | 13 | 52 | Ipsilateral | Middle frontal gyrus | 3.92 | 3.78 | −49 | −54 | 28 | Contralateral | Supramarginal gyrus |
| 3.28 | 3.19 | 9 | −42 | 34 | Ipsilateral | Cingulate gyrus | 3.87 | 3.73 | −55 | 7 | 27 | Contralateral | Inferior frontal gyrus |
| 2.96 | 2.9 | 14 | 31 | −19 | Ipsilateral | Inferior frontal gyrus | 3.74 | 3.61 | −62 | −12 | −1 | Contralateral | Superior temporal gyrus |
| 2.82 | 2.76 | 5 | −3 | −4 | Ipsilateral | Hypothalamus | 3.63 | 3.51 | 23 | −15 | −12 | Ipsilateral | Parahippocampal gyrus |
| 10.43 | Inferior | −33 | −18 | −1 | Contralateral | Putamen | 3.58 | 3.47 | −53 | 7 | 30 | Contralateral | Inferior frontal gyrus |
| 6.02 | 5.59 | −29 | −27 | −12 | Contralateral | Hippocampus | 3.57 | 3.46 | −56 | 7 | 24 | Contralateral | Inferior frontal gyrus |
| 9.09 | 7.76 | −47 | −26 | 0 | Contralateral | Superior temporal gyrus | |||||||
| 3.86 | 3.72 | −49 | 6 | 16 | Contralateral | Inferior frontal gyrus | |||||||
| 3.69 | 3.56 | −44 | 41 | −4 | Contralateral | Inferior frontal gyrus | |||||||
| 3.39 | 3.29 | −23 | 28 | −18 | Contralateral | Inferior frontal gyrus | |||||||
FDR, false discovery rate; ; MTLE, medial temporal lobe epilepsy.
FDR corrected (p<0.05), only clusters >10 voxels
Overall, as shown by fig 2, the number of regions with significant changes in patients with left MTLE was considerably larger than in patients with right MTLE. Therefore, left MTLE is probably associated with more widespread changes than right MTLE.
Effect of duration of epilepsy on synchronous atrophy in left MTLE compared with right MTLE
We found no difference in the mean duration of epilepsy between patients with left and right MTLE (t(34) = 1.6; p = 0.11).
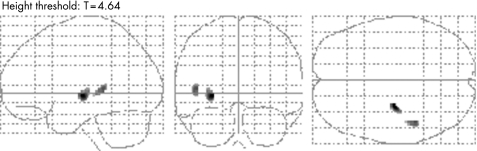
When the effect of duration of refractory epilepsy was excluded, patients with left MTLE showed more intense synchronous atrophy in the contralateral putamen and superior temporal gyrus compared with those with right MTLE (table 4, fig 3).
Table 4 Areas where grey matter is significantly more associated with hippocampal grey matter in patients with left medial temporal lobe epilepsy compared with those with right medial temporal lobe epilepsy, when the effect of duration of epilepsy is excluded from the analysis.
| Correlation with ipsilateral hippocampal grey matter more intense in patients with left MTLE, compared with those with right MTLE, time of symptoms covaried | ||||||
|---|---|---|---|---|---|---|
| Anatomical location | Spatial coordinates | Voxelwise | ||||
| Side | Location | x | y | z | T | z |
| Contralateral | Putamen | −27 | −14 | −1 | 6.3 | 5.04 |
| Contralateral | Superior temporal gyrus | −45 | −35 | 6 | 5.56 | 4.62 |
FDR, false discovery rate; MTLE, medial temporal lobe epilepsy.
Height threshold: T = 2.66, FDR corrected (p<0.05), only clusters >10 voxels.
Figure 3 Areas where the correlation with the atrophied hippocampal grey matter is more intense in patients with left medial temporal lobe epilepsy (MTLE) compared with those with right MTLE, when the effect of duration of epilepsy is excluded. Significant areas, exclusively located in the contralateral hemisphere, are shown overlaid on a “glass brain” template. The hemisphere ipsilateral to hippocampal atrophy is the right hemisphere of the glass brain template.
Discussion
Clinical evidence suggests that refractory unilateral MTLE is different according to the side of hippocampal atrophy and onset of seizures. However, it is not yet clear whether left and right MTLE are different with regard to the intensity and patterns of neuronal loss. The results outlined in this paper suggest that left and right MTLE are associated with unique and different patterns of extra‐hippocampal atrophy in synchrony with hippocampal atrophy. Patients with left MTLE exhibit more intense and widespread neuronal damage than those with right MTLE.
In a previous study, Duzel et al18 observed that patients with MTLE show an intense correlation of grey matter volume in the limbic system with the hippocampal grey matter, suggesting a large network of damage. We have confirmed these findings, but we also hypothesised that the correlation of hippocampal grey matter with limbic grey matter is exhibited by healthy people. Therefore, evaluating the differences between participants with MTLE and controls can discriminate a network of connected atrophy. In this paper, we extracted the grey matter density of the hippocampus. This is possibly a more sensitive way to evaluate the individual profile of grey matter in the hippocampus, compared with the hippocampal volume used by Duzel et al. Therefore, we were able to detect an intense association of grey matter in the limbic system with the hippocampal grey matter in the hippocampus. However, the pattern of hippocampal connectivity, suggested by the extra‐hippocampal grey matter variation related to hippocampal grey matter variation, was different in patients with MTLE compared with controls. Patients with MTLE exhibited a more intense association between grey matter in regions in the limbic system, corroborating the hypothesis of a connected network of atrophy in them.
The findings from this paper also support the empirical notion that left MTLE is associated with a larger degree of symptom severity. For instance, there are differences in neuropsychological performance between patients with left and right MTLE, but thus far there has been little evidence of biological differences between the two groups. Moreover, differences in cognitive performances between the groups may also be a consequence of the different profile of deficits that is characteristic of each group. The functions of the medial temporal lobe are highly lateralised,19 and the classic model of material‐specific memory predicts that lesions in the left hippocampal system impair verbal memory,20,21,22 whereas those in the right hippocampal system affect visual memory.23,24 In patients with MTLE, the relationship between side of hippocampal pathology and categorical memory dysfunction has been suggested to be significantly more intense in patients with left hippocampal atrophy than in those with right hippocampal atrophy.9,25,26,27,28,29,30,31 Patients with left MTLE not only exhibit impairments in memory tasks in the language domain but also perform poorer on tasks that are related to general memory.9 However, it is possible that left MTLE deficits are more prominent because cognitive assessment relies heavily on language‐based tasks. Therefore, an isolated analysis of neuropsychological data might not suffice to conclude that patients with left MTLE are in nature more impaired than those with right MTLE.
Additional evidence for differences between left and right MTLE also comes from preoperative evaluation and surgical outcomes. Kirsch et al32 observed that during the intracarotid amobarbital test, used to evaluate hemispheric memory capacity, most patients show better memory with injection ipsilateral to the hippocampal atrophy (expected asymmetry), but a substantial minority show better memory with contralateral injection (unexpected asymmetry). Interestingly, even though more patients with left MTLE show unexpected asymmetry, the unexpected asymmetry is not associated with worse outcome for patients with left MTLE. In turn, patients with right MTLE are less likely to present unexpected asymmetry, but it is a risk marker of poor surgical outcome. These results possibly indicate that patients with left MTLE are more likely to show a more intense and bilateral compromise of the medial temporal lobes, therefore the temporary anaesthesia of the right medial temporal lobe does not result in a greater difference in terms of memory performance. Likewise, owing to the already compromised function of the temporal lobes, unexpected asymmetry is not predictive of worse outcome for left MTLE. Conversely, right MTLE is possibly less likely to be associated with bilateral compromise.
A large number of studies, using either manual morphometry2,3 or VBM,5,6,7,8,33 have demonstrated that left and right MTLE show a symmetrical pattern of extra‐hippocampal atrophy—that is, the pattern of damage in the ipsilateral lobe is similar in the two groups. Neuronal damage in these patients usually involves the hippocampus, the medial temporal and limbic lobes, the thalami and caudate nuclei, the cerebellum, and frontal and occipital regions. These studies have also consistently noted that, despite the overall symmetry, the intensity of atrophy and regional distribution is not exactly the same for left and right MTLE. Usually, patients with left MTLE show more areas of grey matter reduction compared with controls, than those with right MTLE. For example, a previous study observed that the ipsilateral insula and contralateral occipital lobe were atrophied only in patients with left MTLE.7 In a previous study from our group, we observed that only patients with left MTLE showed atrophy of the ipsilateral frontal operculum, insula, inferior frontal gyrus and superior frontal sulcus.8
A pioneering study also observed that only patients with left MTLE showed reduction of the middle occipital gyrus, angular gyrus, intraparietal sulcus and intraoccipital sulcus, and more bilateral frontal atrophy.5 They also observed that patients with left and right MTLE showed correlation of thalamic grey matter and duration of epilepsy, but patients with left MTLE also exhibited correlation of grey matter in the superior frontal gyrus and precentral gyrus, and time of epilepsy.
Even though these studies hinted at the possibility of differences between atrophy in left and right MTLE, none of them actually conducted a statistical analysis to investigate these differences. It is important to notice that the differences observed could have been accounted for by differences in statistical power, rather than intrinsic regional differences in grey matter loss in left or right MTLE. For example, in one of these studies, when all patients were grouped together (without flipping sides), many structures that were not significantly correlated with time of epilepsy became significant, such as the cingulate gyrus and the middle frontal gyrus, among others.5 It is difficult to determine which side contributes to atrophy in these regions, and if other sites were not shown as significant because of lack of power. A recent hippocampal volumetric study showed that patients with right MTLE may exhibit a more intense correlation between ipsilateral and contralateral hippocampi.34 However, a significant correlation between the two hippocampi was not shown for the two groups of patients combined. In addition, the group of patients studied was more homogeneous than the one from this paper, with respect to strict unilateral pathology and drug refractoriness.
In this paper, we suggest that the side of hippocampal atrophy is relevant to the location of regional extra‐hippocampal grey matter loss: patients with left MTLE exhibit more widespread and intense grey matter loss synchronised with hippocampal atrophy, specifically including the contralateral hippocampus. Possible explanations for these findings include (i) seizures originating in the dominant hemisphere that can cause more excitotoxic damage or (ii) the dominant hemisphere, which is possibly more intensely connected to the rest of the brain, and therefore neuronal loss in the hippocampus may cause neuronal loss from deafferentation in a larger number of remote sites.
Our group recently showed that duration of epilepsy is significantly associated with location and extent of neuronal damage in patients with MTLE.35 Patients with both left and right MTLE included in this paper did not differ with regard to their duration of epilepsy, and we also observed differences in the pattern of synchronous grey matter atrophy related to hippocampal atrophy in patients with left and right MTLE independent of time of epilepsy. Even though covarying out time of symptoms from the analysis can potentially mask some effects, the contralateral putamen and superior temporal gyrus were more atrophied in patients with left MTLE. This suggests that left MTLE is structurally different from right MTLE, at least partly independently of the duration of epilepsy.
It is not clear to what extent the more widespread neuronal loss in patients with left MTLE is a consequence of the effect of seizures originating in the dominant hemisphere, side sensitivity to seizures or deafferentation from a more intensely connected language‐dominant hippocampus. A longitudinal study independently considering the effect of the burden of seizure in these patients is likely to contribute to our understanding of the different variables associated with neuronal loss in patients with left and right MTLE.
Abbreviations
EEG - electroencephalogram
MRI - magnetic resonance imaging
MTLE - medial temporal lobe epilepsy
VBM - voxel‐based morphometry
Footnotes
Competing interests: None declared.
References
- 1.Meencke H J V G. Hippocampal sclerosis in epilepsy. In: Luders HO, ed. Epilepsy surgery. New York: Raven Press, 1991705–715.
- 2.Cendes F, Andermann F, Gloor P.et al MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology 199343719–725. [DOI] [PubMed] [Google Scholar]
- 3.Bonilha L, Kobayashi E, Rorden C.et al Medial temporal lobe atrophy in patients with refractory temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 2003741627–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonilha L, Rorden C, Castellano G.et al Voxel‐based morphometry of the thalamus in patients with refractory medial temporal lobe epilepsy. Neuroimage 2005251016–1021. [DOI] [PubMed] [Google Scholar]
- 5.Keller S S, Wieshmann U C, Mackay C E.et al Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: effects of side of seizure onset and epilepsy duration. J Neurol Neurosurg Psychiatry 200273648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keller S S, Mackay C E, Barrick T R.et al Voxel‐based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage 20021623–31. [DOI] [PubMed] [Google Scholar]
- 7.Bernasconi N, Duchesne S, Janke A.et al Whole‐brain voxel‐based statistical analysis of gray matter and white matter in temporal lobe epilepsy. Neuroimage 200423717–723. [DOI] [PubMed] [Google Scholar]
- 8.Bonilha L, Rorden C, Castellano G.et al Voxel‐based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Arch Neurol 2004611379–1384. [DOI] [PubMed] [Google Scholar]
- 9.Alessio A, Damasceno B P, Camargo C H.et al Differences in memory performance and other clinical characteristics in patients with mesial temporal lobe epilepsy with and without hippocampal atrophy. Epilepsy Behav 2004522–27. [DOI] [PubMed] [Google Scholar]
- 10.Commission on classification and terminology of the International League Against Epilepsy Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 198930389–399. [DOI] [PubMed] [Google Scholar]
- 11.Oldfield R C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971997–113. [DOI] [PubMed] [Google Scholar]
- 12.Spreen O, Strauss E. eds. Language tests. A compendium of neuropsychological tests: administration, norms and commentary. New York: Oxford University Press, 1998423–480.
- 13.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol 200012191–200. [DOI] [PubMed] [Google Scholar]
- 14.Good C D, Johnsrude I S, Ashburner J.et al A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 20011421–36. [DOI] [PubMed] [Google Scholar]
- 15.Salmond C H, Ashburner J, Vargha‐Khadem F.et al Distributional assumptions in voxel‐based morphometry. Neuroimage 2002171027–1030. [PubMed] [Google Scholar]
- 16.Genovese C R, Lazar N A, Nichols T. Thresholding of statistical maps in functional neuroimaging using false discovery rate. Neuroimage 200215870–878. [DOI] [PubMed] [Google Scholar]
- 17.Brett M, Anton J L, Valabregue R.et al Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conference on Functional Mapping of the Human Brain, 2–6 June, 2002, Sendai, Japan. Available on CD Rom in NeuroImage, Vol 16, No 2.
- 18.Duzel E, Schiltz K, Solbach T.et al Hippocampal atrophy in temporal lobe epilepsy is correlated with limbic systems atrophy. J Neurol 2006253294–300. [DOI] [PubMed] [Google Scholar]
- 19.Henson R. A mini‐review of fMRI studies of human medial temporal lobe activity associated with recognition memory. Q J Exp Psychol B 200558340–360. [DOI] [PubMed] [Google Scholar]
- 20.Meyer V, Yate A J. Intellectual changes following temporal lobectomy for psychomotor epilepsy. J Neurol Neurosurg Psychiatry 19551844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milner B. Psychological defects produced by temporal lobe excision. Res Publ Assoc Res Nerv Ment Dis 195836244–257. [PubMed] [Google Scholar]
- 22.Novelly R A, Augustine E A, Mattson R H.et al Selective memory improvement and impairment in temporal lobectomy for epilepsy. Ann Neurol 19841564–67. [DOI] [PubMed] [Google Scholar]
- 23.Milner B, Branch C, Rasmussen T. Study of short‐term memory after intracarotid injection of sodium amytal. Trans Am Neurol Assoc 196287224–226. [Google Scholar]
- 24.Kimura D. Right temporal‐lobe damage. Perception of unfamiliar stimuli after damage. Arch Neurol 19638264–271. [DOI] [PubMed] [Google Scholar]
- 25.Lencz T, McCarthy G, Bronen R A.et al Quantitative magnetic resonance imaging in temporal lobe epilepsy: relationship to neuropathology and neuropsychological function. Ann Neurol 199231629–637. [DOI] [PubMed] [Google Scholar]
- 26.Rausch R, Babb T L. Hippocampal neuron loss and memory scores before and after temporal lobe surgery for epilepsy. Arch Neurol 199350812–817. [DOI] [PubMed] [Google Scholar]
- 27.Saling M M, Berkovic S F, O'Shea M F.et al Lateralization of verbal memory and unilateral hippocampal sclerosis: evidence of task‐specific effects. J Clin Exp Neuropsychol 199315608–618. [DOI] [PubMed] [Google Scholar]
- 28.Trenerry M R, Westerveld M, Meador K J. MRI hippocampal volume and neuropsychology in epilepsy surgery. Magn Reson Imaging 1995131125–1132. [DOI] [PubMed] [Google Scholar]
- 29.Jones‐Gotman M. Psychological evaluation for epilepsy surgery. In: Shorvon S, Dreifuss F, Fish D, Thomas D, eds. The treatment of epilepsy. Oxford: Blackwell Science, 1996621–630.
- 30.Hermann B P, Seidenberg M, Schoenfeld J.et al Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol 199754369–376. [DOI] [PubMed] [Google Scholar]
- 31.Griffith H R, Pyzalski R W, O'Leary D.et al A controlled quantitative MRI volumetric investigation of hippocampal contributions to immediate and delayed memory performance. J Clin Exp Neuropsychol 2003251117–1127. [DOI] [PubMed] [Google Scholar]
- 32.Kirsch H E, Walker J A, Winstanley F S.et al Limitations of Wada memory asymmetry as a predictor of outcomes after temporal lobectomy. Neurology 200565676–680. [DOI] [PubMed] [Google Scholar]
- 33.Keller S S, Wilke M, Wieshmann U C.et al Comparison of standard and optimized voxel‐based morphometry for analysis of brain changes associated with temporal lobe epilepsy. Neuroimage 200423860–868. [DOI] [PubMed] [Google Scholar]
- 34.Garcia‐Finana M, Denby C E, Keller S S.et al Degree of hippocampal atrophy is related to side of seizure onset in temporal lobe epilepsy. Am J Neuroradiol 2006271046–1052. [PMC free article] [PubMed] [Google Scholar]
- 35.Bonilha L, Rorden C, Appenzeller S.et al Gray matter atrophy associated with duration of temporal lobe epilepsy. Neuroimage 2006321070–1079. [DOI] [PubMed] [Google Scholar]