Abstract
Background
Recently, anterior limb of the internal capsule and nucleus accumbens deep brain stimulation (DBS) has been used in the treatment of medication‐refractory obsessive–compulsive disorder (OCD). This region has been previously explored with lesion therapy, but with the advent of DBS there exists the possibility of monitoring the acute and chronic effects of electrical stimulation. The stimulation‐induced benefits and side effects can be reversibly and blindly applied to a variety of locations in this region.
Objective
To explore the acute effects of DBS in the anterior limb of the internal capsule and nucleus accumbens region.
Methods
Ten total DBS leads in five patients with chronic and severe treatment‐refractory OCD were tested. Patients were examined 30 days after DBS placement and received either “sham” testing or actual testing of the acute effects of DBS (the alternative condition tested 30 days later).
Results
Pooled responses were reviewed for comparability of distribution using standard descriptive methods, and relationships between the variables of interest were sought using χ2 analysis. A total of 845 stimulation trials across the five patients were recorded and pooled. Of these 16% were elicited from sham stimulation and 17% from placebo (0 V stimulation). A comparison of active to sham trials showed that sham stimulation was not associated with significant side effects or responses from patients. Non‐mood‐related responses were found to be significantly associated with the ventral lead contacts (0 and 1) (p = 0.001). Responses such as taste, smell and smile were strongly associated with the most ventral lead positions. Similarly, physiological responses—for example, autonomic changes, increased breathing rate, sweating, nausea, cold sensation, heat sensation, fear, panic and panic episodes—were significantly associated with ventral stimulation (p = 0.001). Fear and panic responses appeared clustered around the most ventral electrode (0). Acute stimulation resulted in either improved or worsened mood responses in both the dorsal and ventral regions of the anterior limb of the internal capsule.
Conclusion
The acute effects of DBS in the region of the anterior limb of the internal capsule and nucleus accumbens, particularly when obtained in a blinded fashion, provide a unique opportunity to localise brain regions and explore circuitry.
Recently deep brain stimulation (DBS) in the anterior limb of the internal capsule and nucleus accumbens has been used in the treatment of medication‐refractory obsessive–compulsive disorder (OCD).1,2,3,4 This region has been previously explored with lesion therapy,5,6,7,8,9,10,11,12 but with the advent of DBS there is now a possibility of monitoring the acute and chronic effects of electrical stimulation. The stimulation‐induced benefits and side effects can be reversibly and blindly applied to a variety of locations in this region, which spans 20 mm between proximal and distal contacts on the DBS lead. We report the results of acute blinded DBS in this emerging brain target. The results from this study offer a glimpse at the underlying neural circuitry that may be available for neuromodulation, and the responses seen with acute stimulation.
Methods
Five patients, mean age 38 years (three men and two women), underwent DBS placement in the anterior limb of the internal capsule and nucleus accumbens region (surgical procedure described previously in detail13) for longstanding and severe treatment‐refractory OCD. The mean (standard deviaton (SD)) duration of illness was 17 (4.1) years. All patients were previously unresponsive to comprehensive trials of both medical and cognitive behavioural treatment for OCD.
For each patient, a high‐resolution, volumetric, 3‐T magnetic resonance imaging (MRI) scan was obtained 1 day before the procedure. The morning of the procedure, a Cosman–Roberts–Wells head ring was applied under local anaesthesia and a high‐resolution stereotactic head computed tomography scan was also performed. The computed tomography and MRI images were fused, and the 3‐T MRI images were then used to precisely image the anterior limb of the internal capsule. Image fusion and stereotactic targeting were performed using computer software (ImMerge Zmed) that facilitated targeting in “atlas space”. A Cartesian coordinate system confirmed the patient's mid‐commissural point, and this point was used as a reference to confirm the target. A “larger” DBS lead was used (3387 IES) with 3 mm contacts and 4 mm spacing between contacts. The larger lead was chosen because of the coverage required of this relatively larger target region. The first DBS electrode (in each patient) was placed in the region of the right anterior limb of the internal capsule, with the tip positioned deep and through the anterior commissure and into the approximate centre of the nucleus accumbens. The left‐sided DBS lead (in each patient) was then placed in an identical procedure, with approximate mirror image coordinates to the first. A follow‐up computed tomography–MRI fusion was performed to localise and confirm the final lead positions in all patients.
At approximately 30 days after surgery, the patients were randomised to a staggered (1 month or 2 months) DBS activation as required by the National Institutes of Health Study they consented to. At the visit, patients received either “sham” testing or active testing of the DBS contacts in an effort to define placebo responses. Each patient was studied in two sessions (either one sham/one active or two active). During the sessions across five patients, both leads were tested in each patient. Patients underwent blinded testing during which they sat in a chair behind a curtain (facing a video camera). A DBS programmer fastened a DBS telemetric programming “head” over the patient's impulse generator (anterior chest), and then sat behind a curtain for the duration of the testing. A neurologist specialising in movement disorders stood in front of, and between, the curtain in clear view of the patient and the programmer. Additionally, another member of the team recorded the stimulation states and patient's responses to acute stimulation. The neurologist wrote each change in stimulation parameter on a clipboard visible only to the programmer and note taker. All study personnel were trained to spend identical time with settings, whether they were sham, placebo or active, and to not respond orally or with changes in facial expression to any reaction by the patient. The patient could only see the rating neurologist, who read from a script.
During multiple programming sessions that followed, patients were tested individually at each lead (n = 10) and each contact (0, 1, 2 and 3) at 0, 2, 4, 6 and 8 V (pulse width of 210 μs at each contact) using monopolar DBS. The frequency of stimulation was held constant at 135 Hz. The stimulation amplitude was systematically increased in each patient from the starting value of 0 V, in an attempt to obtain an acute behavioural response that could be recorded. The testing was then repeated (in an identical fashion), first at a pulse width of 90 μs, and then at a pulse width of 450 μs. Slight variations were found in the number of trials performed for patient safety reasons. If the neurologist recorded an unacceptable side effect, then trials of higher amplitudes of stimulation at that contact were aborted.
After the DBS setting was changed, the patient was given 20–30 s and then asked open‐ended questions as to whether there were changes in mood, and/or any perceivable changes or side effects. All responses, including spontaneous responses, were recorded. The neurologist noted in the data whether smiles were elicited by observing the patient during the testing sessions. The digital quantification of smiles has been published previously,13,14 and in this study, we used a visual observation rather than face digitisation. Data were scored according to whether a response could be initiated (at any DBS parameter setting) at specific lead contacts (0, 1, 2 and 3), with the goal of localising which regions elicited responses. At the conclusion of each session (after both leads were tested), the amplitude was titrated to ensure that optimal benefit was reached and that tolerance was evident without side effects. Stimulation was not left on while the contralateral device was tested.
Analysis
Given the preliminary nature of this study and the considerable data on each patient collected during the sham and active DBS testing sessions, analysis was conducted on the pooled stimulation responses from all five patients. Pooled responses were reviewed for comparability of distribution within and between patients using standard descriptive methods. Any relationship between the variables of interest was sought using χ2 analysis (SPSS V.13.0). A Bonferroni correction was completed to adjust for multiple comparisons. Data from the trials were reviewed using χ2 and graphical methods to identify possible “noise” or confounders in the dataset.
Standard definitions of stimulation states were established for the analysis. The first programming session for each patient was either sham (n = 3) or active (n = 2). Patients were blinded to the DBS state. The three patients who had sham DBS were converted to active DBS during their second programming visit. The other two patients continued on active DBS.
Specific definitions for active, placebo and sham DBS were established as follows:
Active DBS: included all responses when patients were receiving stimulation, excluding responses when stimulated at 0 V, unless the response recorded at 0 V was after a high‐voltage response (6–8 volts) and resulted in an effect identical to the 0 V response. As these identical responses at 0 V potentially represented carry‐over responses, they were counted as active rather than placebo for analysis.
Placebo DBS: included all responses recorded during 0 V of an active DBS session (except as clarified above for carry‐over effects).
Sham DBS: included all responses during a completely sham session.
A total of 16% of the total DBS setting trials for this study were sham DBS; 17% of the total DBS setting trials for this study were placebo DBS. Sham and placebo responses were pooled for one analysis when comparing whether sham and placebo were different from active DBS.
Results of testing
Data were recorded and pooled from 10 active stimulation sessions and 5 sham sessions (n = 845 trials) across 5 patients (3 patients received sham DBS and 2 active DBS at initial sessions).
All patients contributed substantially to the dataset (mean 169 (SD 56.8) response trials). No significant differences were noted between the patients across the parameters of lead and voltage. One patient had a greater percentage of active stimulation trials at the pulse width 210 μs, because of tolerance issues.
Sham versus active stimulation
Active stimulation trials comprised 84% of the total dataset, with sham responses comprising 16%. Within the set of active stimulation responses, an additional 17% were considered placebo responses. No significant side effects or active responses were elicited by complete sham stimulation. Analysis of active to sham/placebo responses showed that active stimulation was significantly associated with response across all the measured outcomes (p = 0.001).
All responses in the dataset were obtained from monopolar stimulation with 95% of responses elicited at a frequency of 135 Hz. Laterality of stimulation was equally distributed between the patients and across the stimulation parameters, with 53.7% (95% confidence interval (CI) 50.3 to 57) of trials elicited from the right side of the brain and 46.3% (95% CI 42.9 to 49.6) from the left side. Stimulation trials across the four different lead contact sites (0–3) were equally distributed, with each contact being randomly stimulated in approximately 20% of trials. Voltage parameters (0, 2, 4, 6 and 8 V) and pulse widths (90, 210 and 450 μs) were also similarly represented across the pooled stimulation trials.
Response by lead contact
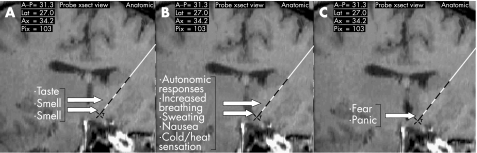
Non‐mood‐related responses (taste, smell and the motor act of smile) were significantly associated with the ventral lead contacts (0 and 1; p = 0.001). The total number of reported smell experiences included metallic (n = 6), odd (n = 10), sweet (n = 4), strange (n = 4) and roses/oil/almonds (n = 13). Tastes included metallic (n = 5), sour (n = 4) and odd (n = 7).
Similarly, other responses including subjectively perceived (by the patient) physiological and physiologically related responses—for example, autonomical changes, increased breathing rate, sweating, nausea, cold sensation, heat sensation, fear, panic and panic episodes—were also significantly associated with ventral stimulation (p = 0.001; table 1). Fear and panic responses appeared clustered around the most ventral electrode (0; fig 1 summarises the contact locations and responses elicited). Two patients in this series had severe clinically relevant panic at the 0 contact.
Table 1 Responses by lead contact (n = 695).
| Response | Lead 0 | Lead 1 | Lead 2 | Lead 3 | Total |
|---|---|---|---|---|---|
| None | 84 | 144 | 126 | 94 | 448 |
| Tired/lethargic | 2 | 4 | 4 | 0 | 10 |
| Heat | 9 | 11 | 2 | 2 | 24 |
| Sweating | 9 | 4 | 1 | 2 | 16 |
| Feeling of increased heart rate | 0 | 2 | 0 | 0 | 2 |
| Nausea | 5 | 0 | 0 | 0 | 5 |
| Cold/chills | 0 | 2 | 4 | 0 | 6 |
| Confusion | 1 | 1 | 3 | 1 | 6 |
| Tingling/chest vibration | 0 | 19 | 15 | 27 | 61 |
| Unclear | 9 | 0 | 1 | 2 | 12 |
| Other—shot up nose, pulling | 9 | 13 | 14 | 7 | 43 |
| Combined | 15 | 13 | 7 | 7 | 42 |
| Fear/extreme distress | 14 | 3 | 0 | 3 | 20 |
| Total | 157 | 216 | 177 | 145 | 695 |
Figure 1 Contact locations and the responses elicited by acute deep brain stimulation. The contacts are defined as 0, ventral (deepest), and then numbered in order (1, 2 and 3) moving dorsally towards the top of the diagram. (A) Non‐mood‐related responses (taste, smell and the motor act of smile) were significantly associated with the ventral lead contacts (0 and 1; p = 0.001). (B) Other responses including physiological and physiologically related responses—for example, autonomic changes, increased breathing rate, sweating, nausea, cold sensation, heat sensation, fear, panic and panic episodes—were also significantly associated with ventral stimulation (p = 0.001). (C) Fear and panic responses appeared clustered around the most ventral electrode (0).
Mood and anxiety responses were significantly associated with lead contact position (p = 0.001). Acute stimulation resulted in either improved or worsened mood responses in both the dorsal and ventral regions of the anterior limb of the internal capsule, as well as in the nucleus accumbens region. However, worsened mood was significantly associated with stimulation of the most ventral lead (p = 0.001), whereas improved mood was more often associated with stimulation of contact 1 of the DBS lead. Reporting of unacceptable side effects by patients was also significantly associated with the two more ventral lead contacts (p = 0.01). No association with laterality of lead was identified.
Response by voltage level
Responses to stimulation at the various voltage levels (0, 2, 4, 6 and 8 V) showed a titration effect. Higher voltage levels showed a significant association with increased sensory (eg, smell and taste) hallucinations (p = 0.001). Physiological responses to acute stimulation across the five voltage levels, although variably distributed, showed a significantly greater range of responses from 6 to 8 V (p = 0.001; 92/375 trials = 24% combined autonomic response to high‐voltage stimulation (6–8 V); 16/301 trials = 5.3% combined autonomic response to low‐voltage stimulation (0–4 V)). Combined autonomic responses (sweating, nausea and panic) were more commonly seen at this level (odds ratio 5.79, 95% CI 3.4 to 9.8).
Mood and anxiety perception was similarly affected by voltage level. Higher voltages were significantly associated with worsening mood and increased anxiety, whereas lower voltages were associated with improved mood and lowered anxiety level (p = 0.001; tables 2 and 3). This result of current change was mirrored by patients' reports of more unacceptable side effects at higher voltage levels (p = 0.001).
Table 2 Subjective mood change by voltage.
| Voltage | Improved mood | Worsened mood | No mood change | Total |
|---|---|---|---|---|
| 0 | 32 | 6 | 102 | 140 |
| 2 | 24 | 16 | 125 | 165 |
| 4 | 31 | 52 | 87 | 170 |
| 6 | 14 | 63 | 51 | 128 |
| 8 | 18 | 35 | 43 | 96 |
| Total | 119 | 172 | 408 | 699 |
Table 3 Subjective anxiety by voltage change.
| Voltage | Anxiety | None | Total |
|---|---|---|---|
| 0 | 9 | 131 | 140 |
| 2 | 10 | 155 | 165 |
| 4 | 45 | 125 | 170 |
| 6 | 58 | 69 | 127 |
| 8 | 43 | 51 | 94 |
| Total | 165 | 531 | 696 |
Response by pulse width
A significant association between pulse width and response was observed from the pooled stimulation data (p = 0.001). The pulse width 210 μs elicited the greatest number and range of responses in comparison to the two other pulse widths. Correspondingly, an increased range of physiological responses was associated with the higher pulse widths 210 and 450 μs (p = 0.001). Autonomic‐related responses (nausea, cold sensation, fear and panic) were recorded more often at these pulse widths. In contrast with physiological responses, no association was found between mood change or anxiety level by either the patient's or the doctor's report, for any pulse width. Likewise, no association was shown between patients' reports of unacceptable side effects and pulse width.
Discussion
The results of this study show several important observations regarding DBS in the anterior limb of the internal capsule and nucleus accumbens region. Firstly, the protocol for this study used a technique that effectively blinded patients to the stimulation condition, and this resulted in consistent and reliable data. The data suggest that effects such as taste, smell, motor smile, autonomic changes, increased breathing rate, sweating, nausea, cold sensation, heat sensation, fear and panic occurred with more ventral stimulation. Further, higher‐amplitude ventral stimulation resulted in more unacceptable side effects. More overall responses occurred at higher voltages and at higher pulse widths. Contact 1 in this study was associated with the greatest mood improvement, and this study showed that although there were mood changes across the full span of the electrode, lower voltages were associated with improvements in mood and anxiety. These data can be used by practitioners charged with programming devices implanted in this region, and by researchers interested in developing an understanding of the underlying neural circuitry.
The data from this study indicated that mood could be improved or worsened by acute stimulation in either the dorsal or ventral regions of the internal capsule, as well as in the nucleus accumbens region. Lower voltages seemed to be better for eliciting positive mood responses. There seemed to be improved mood and giddiness in the more ventral regions. These more ventral regions, when stimulated, may result in a stimulation‐induced smile.13 These results, although not localising, may suggest that broad areas and possibly fibre bundles are involved in these responses. The reasons for the individual responses that were seen, particularly with respect to the plethora of findings with dorsal stimulation, are probably target dependent and lead location dependent. These target and location dependencies have recently been seen in the observations of stimulation‐induced depression, laughter and mania.15,16,17
Swerdlow and Koob18 noted that the nucleus accumbens is at an interface for limbic projections from the amygdala, hippocampus and cingulate cortex. It receives input from dopaminergic‐containing nuclei and may be responsible for mediating behavioural and affective changes of stimulant drugs. Hence, the effects on mood that we observed were possibly mediated by stimulation of the ventral striatum or ventral striatal region. The brain mechanisms that might account for the acute changes seen in these patients are unknown, but there are several possibilities. Haber19 and others20,21,22 have used multiple tracer paradigms to show different striatal regions interface via dopaminergic midbrain cells. These connections form an ascending spiral information flow between multiple striatal regions. They propose a limbic–cognitive–motor interface mediated via the ventral midbrain region. Thus, stimulation of either the ventral striatum (nucleus accumbens region) or ventral and/or dorsal capsular fibres might have elicited limbic responses leading to positive and negative mood changes. Similarly, we have reported that these changes may be partly due to limbic–motor networks.12
Many mood changes were confirmed by this stimulation paradigm. DBS in the region of the nucleus accumbens and the anterior limb of the internal capsule could activate fibres (perhaps descending, but possibly ascending) from the orbital and medial prefrontal cortex and/or cingulate gyrus, which might drive mood changes through one or both of the classic non‐motor basal ganglia loops described by Alexander et al.23,24 Euphoria (a positive change in affective state with the experience of happiness) has been associated with increased activation of the orbitofrontal cortex and medial prefrontal cortex in mood‐induction studies.25,26,27,28 These areas of the frontal lobe are strongly connected to the ventral striatum. Previously, we observed the co‐occurrence of a contralateral smile and euphoria with DBS in this region,12 providing further evidence of limbic and limbic–motor loops, which can be influenced by electrical stimulation.
The fear and panic responses were clustered around the ventral region of the DBS electrode. In particular, DBS in the region of the nucleus accumbens resulted in panic‐like episodes in one patient reported earlier.29 The finding that the ventral contact elicited the strongest response and that this response was reproducible and reliable (not elicited by sham stimulation) may help clarify the potential circuits responsible for fear, panic and panic episodes. Hypothalamic and/or autonomic fibres in the same circuitry may mediate the increase in heart rate, flushing and heat sensation observed in our patients (and also seen with ventral stimulation), but this topic remains open for future investigations.
Recent data indicate that DBS in the subthalamic nucleus can both impair fear recognition30 and induce fear and panic.31,32 It may be hypothesised that we activated the amygdala in a manner similar to DBS in the subthalamic nucleus region. In this manner, the panic responses elicited may be the result of activation of efferent fibres that may emanate from many different regions of the brain, but that all connect to the amygdala and ultimately the frontal cortex.
Finally, the other effects of DBS evidenced in this case can be explained on the basis of lead location and surrounding neuroanatomy, especially as they occurred predominantly in the ventral region of electrical stimulation. The olfactory and gustatory hallucinations probably resulted from current spread anterior to the DBS lead into the region of the medial olfactory stria, and perhaps as the result of stimulation of olfactory and gustatory fibres in the anterior commissure.33,34 The nausea was probably the result of current spread into the anterior hypothalamus, or into pathways involving the temporal lobe cortex.35 The sweating, heat sensation, cold sensation, increased heart rate and increased breathing may have resulted from activation of autonomic fibres perhaps associated with the hypothalamus. Although the exact mechanisms remain to be elucidated, the circumscribed region over which these effects occurred helps us to narrow the mechanistic possibilities.
The acute effects of DBS in the region of the anterior limb of the internal capsule and nucleus accumbens, particularly when obtained in a blinded fashion, provide us with a unique opportunity to localise brain regions that may respond therapeutically to this treatment modality. Understanding the circuitry of these regions could lead to more focused treatments. This study has shown a blinding technique that can be used to reliably sort patients' responses and improve the validity of results. Future studies, however, need to improve on the methodological limitations of this report by testing each condition for a longer period of time, focusing on specific regions or specific symptoms, and using functional imaging to help localise the circuits involved in particular changes. This study did not seek to examine the chronic effects of DBS or habituation of responses. Despite the limitations of this study, it does provide a “snapshot” of the contents of the anterior limb of the internal capsule and nucleus accumbens, and future data may help guide clinician programmers as well as brain‐behaviour researchers. We hope to deal with the potential relevance of acute responses to predicting clinical effects in follow‐up studies.
Abbreviations
DBS - deep brain stimulation
MRI - magnetic resonance imaging
OCD - obsessive–compulsive disorder
Footnotes
Competing interests: None declared.
References
- 1.Nuttin B J, Gabriels L A, Cosyns P R.et al Long‐term electrical capsular stimulation in patients with obsessive‐compulsive disorder. Neurosurgery 2003521263–1274. [DOI] [PubMed] [Google Scholar]
- 2.Cosyns P, Gabriels L, Nuttin B. Deep brain stimulation in treatment refractory obsessive compulsive disorder. Verh K Acad Geneeskd Belg 200365385–400. [PubMed] [Google Scholar]
- 3.Gabriels L, Cosyns P, Nuttin B.et al Deep brain stimulation for treatment‐refractory obsessive‐compulsive disorder: psychopathological and neuropsychological outcome in three cases. Acta Psychiatr Scand 2003107275–282. [PubMed] [Google Scholar]
- 4.Abelson J L, Curtis G C, Sagher O.et al Deep brain stimulation for refractory obsessive‐compulsive disorder. Biol Psychiatry 200557510–516. [DOI] [PubMed] [Google Scholar]
- 5.Lippitz B, Mindus P, Meyerson B A.et al Obsessive compulsive disorder and the right hemisphere: topographic analysis of lesions after anterior capsulotomy performed with thermocoagulation. Acta Neurochir Suppl (Wien) 19976861–63. [DOI] [PubMed] [Google Scholar]
- 6.Irle E, Exner C, Thielen K.et al Obsessive‐compulsive disorder and ventromedial frontal lesions: clinical and neuropsychological findings. Am J Psychiatry 1998155255–263. [DOI] [PubMed] [Google Scholar]
- 7.Kim M C, Lee T K, Choi C R. Review of long‐term results of stereotactic psychosurgery. Neurol Med Chir (Tokyo) 200242365–371. [DOI] [PubMed] [Google Scholar]
- 8.Cosgrove G R, Rauch S L. Stereotactic cingulotomy. Neurosurg Clin N Am 200314225–235. [DOI] [PubMed] [Google Scholar]
- 9.Montoya A, Weiss A P, Price B H.et al Magnetic resonance imaging‐guided stereotactic limbic leukotomy for treatment of intractable psychiatric disease. Neurosurgery 2002501043–1052. [DOI] [PubMed] [Google Scholar]
- 10.Dougherty D D, Baer L, Cosgrove G R.et al Prospective long‐term follow‐up of 44 patients who received cingulotomy for treatment‐refractory obsessive‐compulsive disorder. Am J Psychiatry 2002159269–275. [DOI] [PubMed] [Google Scholar]
- 11.Rauch S L, Dougherty D D, Cosgrove G R.et al Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for obsessive compulsive disorder. Biol Psychiatry 200150659–667. [DOI] [PubMed] [Google Scholar]
- 12.Baer L, Rauch S L, Ballantine H T., Jret al Cingulotomy for intractable obsessive‐compulsive disorder. Prospective long‐term follow‐up of 18 patients. Arch Gen Psychiatry 199552384–392. [DOI] [PubMed] [Google Scholar]
- 13.Okun M S, Bowers D, Springer U.et al What's in a “smile?” Intra‐operative observations of contralateral smiles induced by deep brain stimulation. Neurocase 200410271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Springer U S, Bowers D, Goodman W K.et al Long‐term habituation of the smile response with deep brain stimulation. Neurocase 200612191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bejjani B P, Damier P, Arnulf I.et al Transient acute depression induced by high‐frequency deep‐brain stimulation. N Engl J Med 19993401476–1480. [DOI] [PubMed] [Google Scholar]
- 16.Krack P, Kumar R, Ardouin C.et al Mirthful laughter induced by subthalamic nucleus stimulation. Mov Disord 200116867–875. [DOI] [PubMed] [Google Scholar]
- 17.Bejjani B P, Houeto J L, Hariz M.et al Aggressive behavior induced by intraoperative stimulation in the triangle of Sano. Neurology 2002591425–1427. [DOI] [PubMed] [Google Scholar]
- 18.Swerdlow N R, Koob G F. Dopamine, schizophrenia, mania and depression: toward a unified hypothesis of cortico‐striatal‐pallido‐thalamic function. Behav Brain Sci 198710197–245. [Google Scholar]
- 19.Haber S N. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 200326317–330. [DOI] [PubMed] [Google Scholar]
- 20.McFarland N R, Haber S N. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci 2002228117–8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fudge J L, Kunishio K, Walsh P.et al Amygdaloid projections to ventromedial striatal subterritories in the primate. Neuroscience 2002110257–275. [DOI] [PubMed] [Google Scholar]
- 22.Haber S, McFarland N R. The place of the thalamus in frontal cortical‐basal ganglia circuits. Neuroscientist 20017315–324. [DOI] [PubMed] [Google Scholar]
- 23.Alexander G E, Crutcher M D, DeLong M R. Basal ganglia‐thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 199085119–146. [PubMed] [Google Scholar]
- 24.Alexander G E, DeLong M R, Strick P L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 19869357–381. [DOI] [PubMed] [Google Scholar]
- 25.Gosain A K, Amarante M T, Hyde J S.et al A dynamic analysis of changes in the nasolabial fold using magnetic resonance imaging: implications for facial rejuvenation and facial animation surgery. Plast Reconstr Surg 199698622–636. [DOI] [PubMed] [Google Scholar]
- 26.Gosain A K, Birn R M, Hyde J S. Localization of the cortical response to smiling using new imaging paradigms with functional magnetic resonance imaging. Plast Reconstr Surg 20011081136–1144. [DOI] [PubMed] [Google Scholar]
- 27.Iwase M, Ouchi Y, Okada H.et al Neural substrates of human facial expression of pleasant emotion induced by comic films: a PET Study. Neuroimage 200217758–768. [PubMed] [Google Scholar]
- 28.O'Doherty J, Winston J, Critchley H.et al Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia 200341147–155. [DOI] [PubMed] [Google Scholar]
- 29.Shapira N A, Okun M S, Wint D.et al Panic and fear induced by deep brain stimulation. J Neurol Neurosurg Psychiatry 200677410–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biseul I, Drapier S, Saleau P.et al Fear recognition is impaired by subthalamic nucleus stimulation in Parkinson's disease. Mov Disord 200419292 [Google Scholar]
- 31.Okun M S, Green J, Saben R.et al Mood changes with deep brain stimulation of STN and GPi: results of a pilot study. J Neurol Neurosurg Psychiatry 2003741584–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okun M S, Raju D V, Walter B L.et al Pseudobulbar crying induced by stimulation in the region of the subthalamic nucleus. J Neurol Neurosurg Psychiatry 200475921–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohman A H, Mentink G M. The lateral olfactory tract, the anterior commissure and the cells of the olfactory bulb. Brain Res 196912396–413. [DOI] [PubMed] [Google Scholar]
- 34.Slotnick B M, Schoonover F W. Olfactory pathways and the sense of smell. Neurosci Biobehav Rev 199216453–472. [DOI] [PubMed] [Google Scholar]
- 35.Hornby P J. Central neurocircuitry associated with emesis. Am J Med 2001111(Suppl 8A)106S–112S. [DOI] [PubMed] [Google Scholar]