Abstract
Immunological methods were used to examine the flagellin production of Salmonella typhimurium strains that carried a mutation in one of the two possible genes for flagellin (H1 or H2) and also were incapable of expressing the other gene. Some mutants produced flagellin that was excreted into the culture medium; others accumulated flagellin intracellularly. These two phenotypes were detected in both H1 and H2 mutants. The mutation sites were mapped on the corresponding deletion map (consisting of 21 segments in the case of H1 and 31 segments in the case of H2). H1 and H2 mutations causing excretion of flagellin were clustered mainly in segment 12 and segment 6 from the proximal end, respectively, suggesting that the corresponding segments of the flagellins play a role in polymerization. Mutations causing accumulation in the cytoplasm were clustered in segments 19 to 21 of the H1 map and in segments 25 to 29 of the H2 map, suggesting that an essential region for flagellin transport exists toward the C terminus of flagellin.
Full text
PDF

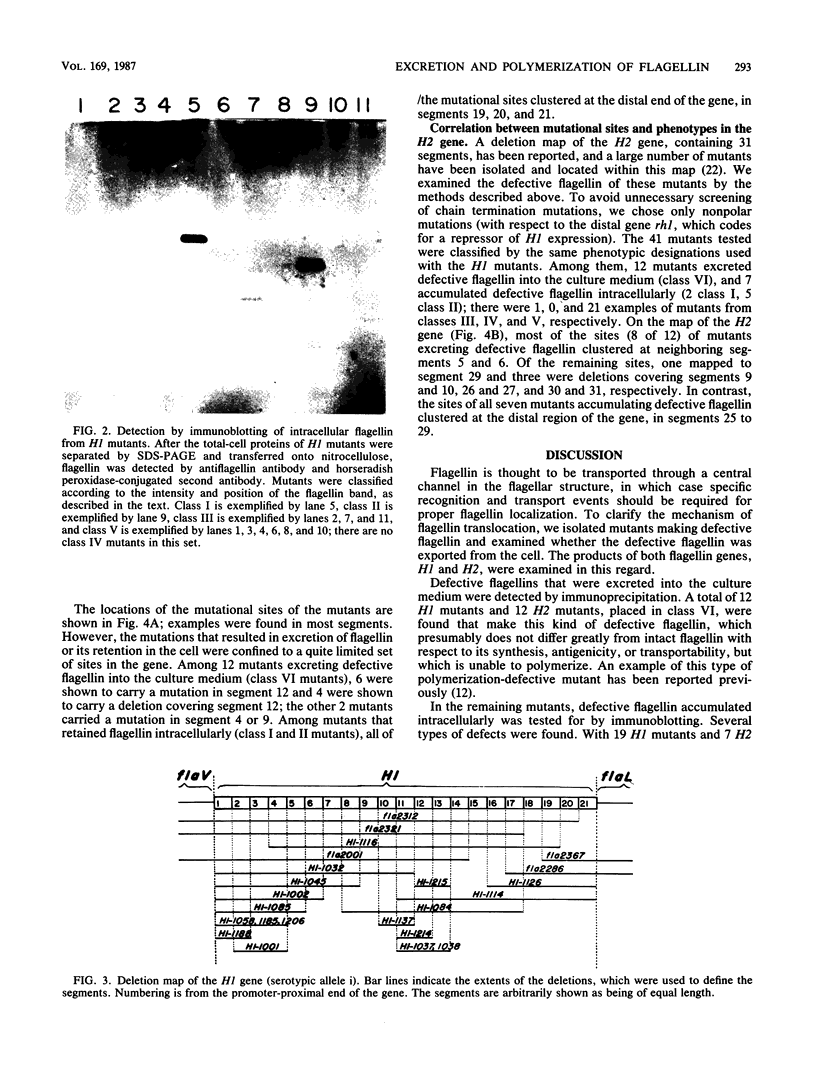
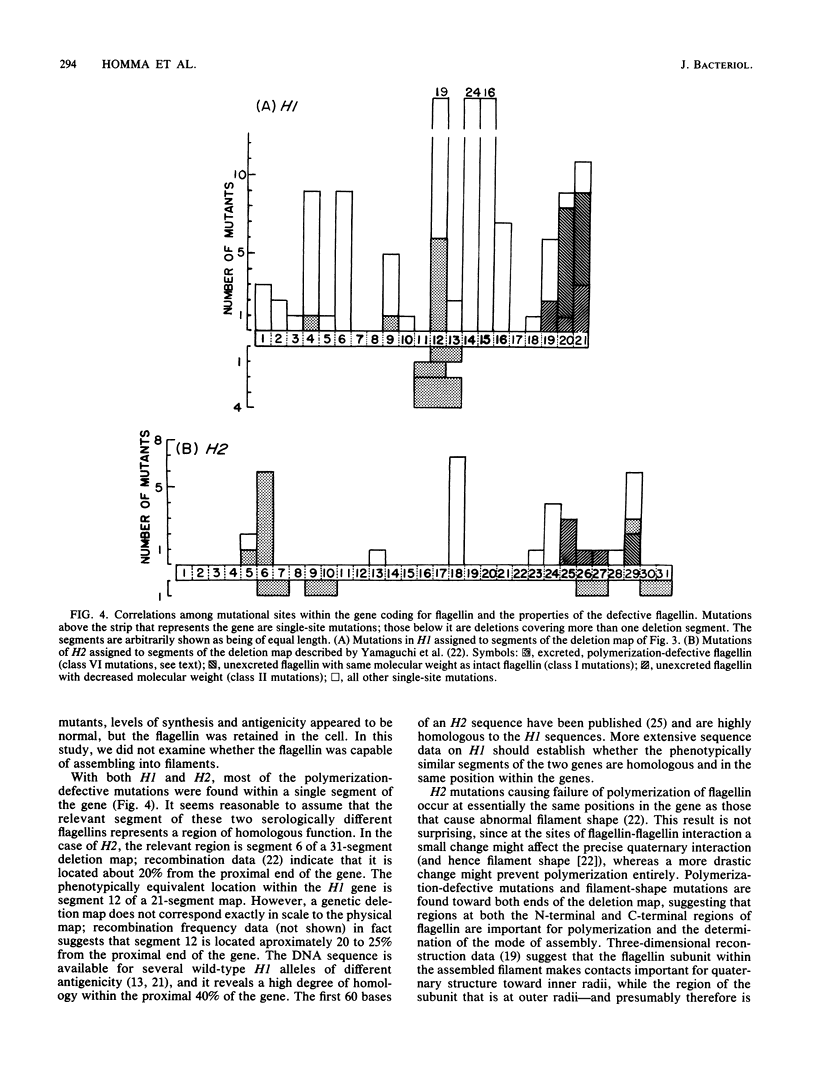


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Emerson S. U., Tokuyasu K., Simon M. I. Bacterial flagella: polarity of elongation. Science. 1970 Jul 10;169(3941):190–192. doi: 10.1126/science.169.3941.190. [DOI] [PubMed] [Google Scholar]
- Fujita H., Yamaguchi S., Taira T., Iino T. A simple method for the isolation of flagellar shape mutants in Salmonella. J Gen Microbiol. 1981 Jul;125(1):213–216. doi: 10.1099/00221287-125-1-213. [DOI] [PubMed] [Google Scholar]
- Homma M., Fujita H., Yamaguchi S., Iino T. Excretion of unassembled flagellin by Salmonella typhimurium mutants deficient in hook-associated proteins. J Bacteriol. 1984 Sep;159(3):1056–1059. doi: 10.1128/jb.159.3.1056-1059.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Iino T. Excretion of unassembled hook-associated proteins by Salmonella typhimurium. J Bacteriol. 1985 Dec;164(3):1370–1372. doi: 10.1128/jb.164.3.1370-1372.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Iino T. Locations of hook-associated proteins in flagellar structures of Salmonella typhimurium. J Bacteriol. 1985 Apr;162(1):183–189. doi: 10.1128/jb.162.1.183-189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Kutsukake K., Iino T. Structural genes for flagellar hook-associated proteins in Salmonella typhimurium. J Bacteriol. 1985 Aug;163(2):464–471. doi: 10.1128/jb.163.2.464-471.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Kutsukake K., Iino T., Yamaguchi S. Hook-associated proteins essential for flagellar filament formation in Salmonella typhimurium. J Bacteriol. 1984 Jan;157(1):100–108. doi: 10.1128/jb.157.1.100-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino T. Assembly of Salmonella flagellin in vitro and in vivo. J Supramol Struct. 1974;2(2-4):372–384. doi: 10.1002/jss.400020226. [DOI] [PubMed] [Google Scholar]
- Iino T. Polarity of flagellar growth in salmonella. J Gen Microbiol. 1969 May;56(2):227–239. doi: 10.1099/00221287-56-2-227. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Kamiya R., Yamaguchi S. Excretion of flagellin by a short-flagella mutant of Salmonella typhimurium. J Bacteriol. 1983 Jan;153(1):506–510. doi: 10.1128/jb.153.1.506-510.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda Y., Silverman M., Simon M. Identification of the structural gene for the hook subunit protein of Escherichia coli flagella. J Bacteriol. 1978 Jan;133(1):364–371. doi: 10.1128/jb.133.1.364-371.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Brien E. J., Bennett P. M. Structure of straight flagella from a mutant Salmonella. J Mol Biol. 1972 Sep 14;70(1):133–152. doi: 10.1016/0022-2836(72)90168-4. [DOI] [PubMed] [Google Scholar]
- Shirakihara Y., Wakabayashi T. Three-dimensional image reconstruction of straight flagella from a mutant Salmonella typhimurium. J Mol Biol. 1979 Jul 5;131(3):485–507. doi: 10.1016/0022-2836(79)90004-4. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Iino T. In vitro synthesis of phase-specific flagellin of Salmonella. J Mol Biol. 1973 Nov 25;81(1):57–70. doi: 10.1016/0022-2836(73)90247-7. [DOI] [PubMed] [Google Scholar]
- Wei L. N., Joys T. M. Covalent structure of three phase-1 flagellar filament proteins of Salmonella. J Mol Biol. 1985 Dec 20;186(4):791–803. doi: 10.1016/0022-2836(85)90397-3. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Fujita H., Taira T., Kutsukake K., Homma M., Iino T. Genetic analysis of three additional fla genes in Salmonella typhimurium. J Gen Microbiol. 1984 Dec;130(12):3339–3342. doi: 10.1099/00221287-130-12-3339. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Iino T., Horiguchi T., Ota K. Genetic analysis of fla and mot cistrons closely linked to H1 in Salmonella abortusequi and its derivatives. J Gen Microbiol. 1972 Apr;70(1):59–75. doi: 10.1099/00221287-70-1-59. [DOI] [PubMed] [Google Scholar]
- Zieg J., Simon M. Analysis of the nucleotide sequence of an invertible controlling element. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4196–4200. doi: 10.1073/pnas.77.7.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]