Abstract
Objective
To relate cerebral perfusion abnormalities to subsequent changes in clinical status among patients with mild cognitive impairment (MCI).
Methods
Perfusion single photon emission computed tomography (SPECT) images were acquired in 105 elderly patients without dementia with MCI, using 99mTc‐HMPAO. Clinical outcome after a 5‐year follow‐up period was heterogeneous.
Results
Baseline SPECT data differed in those patients with MCI who were later diagnosed with Alzheimer's disease (the converter group) from those patients with MCI who experienced clinically evident decline but did not progress to a diagnosis of Alzheimer's disease within the follow‐up period (the decliner group), from patients with MCI who had no clinical evidence of progression (the stable group), and from a group of 19 normal subjects (the control group). The most consistent decreases in relative perfusion in converters compared with the normal, stable and decliner groups were observed in the caudal anterior cingulate, and in the posterior cingulate. In addition, converters showed increased relative perfusion in the rostral anterior cingulate in comparison to the stable and decliner groups. A group of patients with Alzheimer's disease were also included for purposes of comparison. The group of patients with Alzheimer's disease at baseline differed from each of the other groups, with temporoparietal regions showing the most significant reductions in perfusion.
Conclusions
These results suggest that clinical heterogeneity in MCI is reflected in SPECT perfusion differences, and that the pattern of perfusion abnormalities evolves with increasing clinical severity.
Regional abnormalities in glucose metabolism and cerebral perfusion are known to occur in patients with a diagnosis of probable Alzheimer's disease, based on positron emission tomography (PET) and single photon emission computed tomography (SPECT). Decreased metabolism and/or perfusion has been reported primarily in temporoparietal, posterior cingulate and medial temporal regions, and such abnormalities seem to reflect the severity and progression of both clinical impairment and pathological involvement.1,2,3,4,5
Identification of Alzheimer's disease at the earliest possible time is crucial for optimal care and treatment. Therefore, recent studies have focused on prodromal Alzheimer's disease. This has been studied by examining baseline PET or SPECT images in patients who are at increased risk for developing Alzheimer's disease because of mild cognitive impairment, and who then go on to be diagnosed with probable Alzheimer's disease. Brain regions reported to show metabolism or perfusion abnormalities in those who progress to Alzheimer's disease have included the temporoparietal neocortex, posterior cingulate, anterior cingulate and medial temporal lobe regions.3,6,7,8,9,10,11,12,13,14,15
Few of these studies have compared those patients with MCI who will progress over the next few years to the point where they satisfy the diagnostic criteria for probable Alzheimer's disease with those who decline to a more limited extent and are not diagnosed with Alzheimer's disease and with those who remain stable. Only one previous study, to our knowledge, has dealt with this issue.8 The authors reported that left temporoparietal reductions in glucose metabolism, in combination with performance on a neuropsychological task (ie, block design), considerably discriminated those people with memory problems who developed Alzheimer's disease within 3 years from those who remained stable. This study targeted a small number of brain regions for examination, as sample size was limited.
We dealt with this question by examining whole brain SPECT datasets using statistical parametric mapping (SPM) in a large number of subjects, some of whom were normal and some of whom had mild cognitive impairments but did not have dementia when the data were acquired. The subjects were then followed longitudinally, and we were able to identify perfusion differences at baseline between subjects who progressed to a diagnosis of Alzheimer's disease, versus those who remained stable, and those who declined, but were not diagnosed with Alzheimer's disease during the follow‐up interval. We also determined whether the changes were consistently decreased in cerebral perfusion, or whether increases were also observed, as reported by a recent study.14 Lastly, we examined the relationship between SPECT perfusion measures and neuropsychological test scores in the same subjects.
Methods
Selection of participants
The participants in the study consisted of 158 older individuals. Of these, 125 were participants in a longitudinal study examining the incipient stages of Alzheimer's disease.16 The primary goal of the study as a whole was the examination of cognitive, brain structure/function and genetic factors involved in the prodromal phase of Alzheimer's disease. An additional group of 34 subjects with a clinical diagnosis of probable Alzheimer's disease was included in the present analyses to enable direct comparison of data from mildly impaired subjects without dementia with data from patients with mild Alzheimer's disease, using the same analytical methods.
The participants in the longitudinal study had been recruited through the print media (rather than from a clinical or other medical referral source). The advertisements for participants indicated that a research study was seeking subjects both with and without memory difficulty.
Volunteers who responded to the advertisements then underwent a multistage screening procedure. Details of the screening procedures have been described elsewhere.16 Briefly, to be included in the study, participants had to be ⩾65 years old (with the exception of seven individuals 57–64 years old), had to have an informant who could provide information about their daily function, had to be free of significant underlying medical, neurological or psychiatric illness, and had to be willing to participate in the study procedures. In addition, individuals with evidence of major vascular risk factors (eg, atrial fibrillation, insulin‐dependent diabetes, cerebral infarcts, etc) were excluded. All subjects were required to be either cognitively normal or mildly impaired, but without dementia—that is, had to have a Clinical Dementia Rating (CDR) of either 0 or 0.5.17
The patients with Alzheimer's disease were recruited from clinical sources. The diagnosis of Alzheimer's disease was based on a neurological, psychiatric and neuropsychological evaluation. The patients, who ranged in age from 64 to 80 years, met the National Institute of Neurological Disorders and Stroke/Alzheimer's Disease and Related Disorders Association criteria for probable Alzheimer's disease.18 To test for the criteria, standard laboratory tests were used to screen for various neoplastic, infectious or metabolic causes for dementia. Individuals with a history of medical conditions associated with dementia were excluded, including those with a record of severe head trauma, alcoholism or serious psychiatric illness. All patients with Alzheimer's disease received an ischaemic score of ⩽4 on the Ischaemic Scale for assessing the likelihood of multi‐infarct dementia.19
The study procedures performed at baseline included a medical evaluation (ie, medical history and physical examination, ECG and standard laboratory tests), a semistructured interview, neuropsychological testing, magnetic resonance imaging (MRI) and SPECT scans, and blood test for genetic analysis. Only the semistructured interview was repeated annually; the remaining study procedures were repeated in subsets of participants. All participants provided informed consent before the initiation of the study, in accordance with the guidelines of the Massachusetts General Hospital, Boston, Massachusetts, USA.
Assessment of clinical severity
The degree of clinical severity of the participants was evaluated by the annual semistructured interview. This interview generates both an overall CDR rating and a measure known as the CDR Sum of Boxes (CDR‐SB). The interview was based on the Initial Subject Protocol that was used in the development of the CDR scale.17 It includes a set of questions regarding functional status asked of the subject and a collateral source (eg, family member or friend), and a standardised neurological, psychiatric and mental status evaluation of the participant. To be sensitive to clinical impairments at the mildest end of the spectrum, a special set of questions was added to the interview, and the reliability and validity of the revised interview were examined.20 The mean inter‐rater reliability of the CDR ratings in the context of the present study was high (r = 0.99, p<0.001), as was the inter‐rater reliability of the six CDR subcategories (r = 0.90) that were used to generate the overall CDR rating.20 The CDR‐SB represents the sum of the ratings in each of the six CDR subcategories; thus, the inter‐rater reliability for this measure was also high. In the current study, each interview was conducted by a masters or doctoral level clinician (eg, psychiatrist, neuropsychologist or physician's assistant), and was performed without knowledge of the other study procedures, including the neuropsychological testing or the SPECT findings. The interview took approximately 1–2 h to complete.
Group characteristics at baseline and at follow‐up
Baseline
On the basis of their initial CDR interview, participants in the longitudinal study were divided into two groups. The normal group consisted of 19 people with normal cognition (CDR = 0); their mean CDR‐SB score was 0. The group with mild cognitive impairment consisted of 105 individuals without dementia, with a CDR rating of 0.5; their mean CDR‐SB was 0.96 (SD 0.68). The two groups were similar in age, education and mean Mini‐Mental State Examination (MMSE) score at study entry21 (table 1). The sex distribution differed slightly among the groups. The mean MMSE score of the patients with Alzheimer's disease was significantly different from that of the participants in the longitudinal study (table 1).
Table 1 Mean age, sex, education, Clinical Dementia Rating Sum of Boxes and Mini‐Mental State Examination scores for each of the groups at baseline.
| Group | n | Age Mean (SD) | Sex (M:F) | Education (years) Mean (SD) | CDR‐SB Mean (SD) | MMSE Mean (SD) |
|---|---|---|---|---|---|---|
| Normal | 19 | 73.1 (3.6) | 6:13 | 14.3 (2.5) | 0 (0) | 29.3 (0.7) |
| Stable | 38 | 73.2 (4.8) | 19:19* | 15.8 (2.8) | 0.84 (0.45) | 29.5 (0.7) |
| Decliner | 43 | 73.3 (4.9) | 13:30 | 14.9 (3.1) | 0.71 (0.60) | 29.3 (0.9) |
| Converter | 24 | 75.0 (5.2) | 10:14 | 15.1 (2.7) | 1.60 (0.72) | 28.7 (1.6) |
| AD | 34 | 75.1 (3.9) | 8:26† | NA | NA | 15.6 (6.3)† |
| Total | 158 | 73.9 (4.6) | 57:102 |
AD, Alzheimer's disease; CDR‐SB, Clinical Dementia Rating Sum of Boxes; F, female; M, male; MMSE, Mini‐Mental State Examination; NA, not available.
*χ2 = 5.37; p<0.05.
†t test p<0.001.
Table 1 shows that the distribution of CDR‐SB scores among the mildly impaired subjects was broad. At the mild end of the spectrum (ie, CDR‐SB = 0.5−1.5), many patients would not meet psychometric cut‐offs commonly used to select subjects with MCI in epidemiological studies and clinical trials.22,23 The subjects at the more impaired end of the spectrum (ie, CDR‐SB ⩾2) are comparable to those with MCI recruited from these settings, based on the likelihood of progression to a diagnosis of Alzheimer's disease.20 We use MCI here to refer to the entire group of mildly impaired subjects.
We did not use the neuropsychological test scores of the subjects to determine group status at baseline. However, for the purposes of this report, we conducted a retrospective examination of the baseline neuropsychological test performance of the patients in order to determine whether they met the clinical criteria for MCI at baseline,24 based on a combination of clinical history and a score of 1 SD below the mean of controls for each cognitive measure. This review indicated that 103 of the 105 patients with a CDR of ⩾0.5 met the clinical criteria for MCI at baseline. Of these 103 individuals, 68% (n = 70) met the criteria for amnestic MCI and 32% (n = 33) met the criteria for non‐amnestic MCI. Of those with amnestic MCI, 59% (n = 41) met the criteria for amnestic MCI, multiple domains impaired, whereas the remainder (n = 29) met the criteria for amnestic MRI, single domain impaired.
Follow‐up
On follow‐up, participants in the longitudinal study were classified into one of four groups, based on the level and change in the CDR‐SB over 4 years of follow‐up:
Group 1—the normal group (n = 19), consisted of subjects who had CDR‐SB scores = 0 at baseline and throughout the follow‐up period.
Group 2—the stable group (n = 38), had no increase in CDR‐SB scores during the follow‐up period.
Group 3—the decliner group (n = 43), had CDR‐SB scores that increased during the follow‐up period, but the subjects were clinically judged to be without dementia, and CDR‐SB scores were <3.5 at the last follow‐up evaluation.
Group 4—the converter group (n = 24), had CDR‐SB scores that increased to ⩾4 and were diagnosed with probable Alzheimer's disease, as described below.
Group 5—the Alzheimer's disease group (n = 34), consisted of patients who met the criteria for probable Alzheimer's disease at baseline; as noted above, these patients were not participants in the longitudinal study.
Table 1 provides details concerning the age, sex, CDR‐SB and MMSE scores of the groups.21
MCI diagnosis for follow‐up groups
On the basis of the retrospective review of the cases mentioned above, we also determined the number of participants in each of the follow‐up groups who met the criteria for MCI at baseline. Of the 38 participants who remained stable over time, 37% (n = 14) met criteria for non‐amnestic MCI at baseline, and 63% (n = 24) met the criteria for amnestic MCI; of those with amnestic MCI, 50% (n = 12) met the criteria for amnestic MCI, multiple domains impaired, and 50% (n = 12) met the criteria for amnestic MCI, single domain impaired. Of the 43 decliners, 30% (n = 13) met the criteria for non‐amnestic MCI at baseline and 65% (n = 28) met the criteria for amnestic MCI at baseline; of those with amnestic MCI, 50% (n = 14) met the criteria for amnestic MCI, multiple domains impaired, and 50% (n = 14) met the criteria for amnestic MCI, single domain impaired (two decliners (5% of the total) did not meet the criteria for MCI at baseline). Of the 24 converters, 25% (n = 6) met the criteria for non‐amnestic MCI at baseline and 75% (n = 18) met the criteria for amnestic MCI at baseline; of those with amnestic MCI, 62% (n = 15) met the criteria for amnestic MCI, multiple domains impaired, and 12% (n = 3) met the criteria for amnestic MCI, single domain impaired. Thus, the converters had a much larger proportion of individuals with amnestic MCI multiple domains impaired than either of the other two groups.
Diagnosis of dementia on follow‐up
Study participants who showed increased clinical severity over time received a consensus diagnosis to determine: (1) whether they had sufficient impairment for a diagnosis of dementia, and, if so, (2) whether the dementia was consistent with research criteria for Alzheimer's disease18 or another known diagnostic entity (eg, frontotemporal dementia, multi‐infarct dementia, etc). Diagnoses were based on a combination of clinical history, medical records, laboratory evaluation and structural imaging studies used for the purposes of identifying a structural lesion or other vascular pathology. All subjects with dementia who were not clinically diagnosed with Alzheimer's disease were excluded from the group of converters in the current analyses.
Autopsy findings
Over the course of the longitudinal study, 15 subjects have undergone autopsy, of whom 9 had a clinical diagnosis of probable Alzheimer's disease, which was confirmed in 6 of the cases, based on the National Institute of Aging–Reagan Institute criteria for Alzheimer's disease. An additional three cases showed Alzheimer's disease changes insufficient for an autopsy diagnosis of definite Alzheimer's disease.
SPECT image acquisition
SPECT images were acquired 20 min after intravenous injection of 20 (SD 1) mCi of 99mTc‐HMPAO (Ceretec, Amersham, UK), injected into a previously placed intravenous line with the subject supine at rest, with eyes open, in a darkened room and with ambient white noise. SPECT images were obtained with the Ceraspect brain camera (Digital Scintigraphics, Waltham, Massachusetts, USA), which consists of an annular sodium iodide crystal and a rotating parallel hole collimator. Spatial resolution is 8.2 mm fullwidth at half‐maximum at the centre of the field of view for 99mTc‐HMPAO.25 The acquisition time was 15 s per projection for a total acquisition time of 30 min. Datasets were corrected for attenuation and reconstructed with filtered backprojection, using a Butterworth filter.
Statistical analysis of SPECT data
Voxel‐based analyses
SPECT data were analysed using Statistical Parametric Mapping (SPM2, Wellcome Department of Cognitive Neurology, Functional Imaging Laboratory, London, UK).26 Each subject's dataset was spatially transformed to the SPM template SPECT image and smoothed with a 10 mm full‐width at half‐maximum Gaussian kernel. To minimise edge effects, voxels with values <80% of the whole brain mean were excluded.
For the primary analyses, voxel values were proportionally scaled to the mean global voxel value. An alternate method for normalisation in the SPM analyses was also examined to determine the stability of the primary set of findings. For this method, activity within the cerebellum was used to normalise the data. To calculate cerebellar activity, activity density was measured within cerebellar regions of interest (ROIs) for each data‐set, using the SPM ROI toolbox MarsBaR (MARSeille Boite A Region; www.mrccbu.cam.ac.uk/Imaging/marsbar.html).27
One‐way analysis of variance (ANOVA) was used to examine differences across groups, and post hoc a priori pairwise comparisons were used for between‐group analyses (two‐sample t tests converted to z scores). Each pairwise analysis was performed in both positive and negative directions (ie, contrasts 1, −1 and −1, 1), to observe changes in relative perfusion that were either decreases or increases in the second group compared with the first. The threshold for significance at the voxel level was set at p<0.05, corrected for multiple comparisons using the false discovery rate method.28 For purposes of comparison across normalisation procedures, these analyses were repeated using the cerebellum as a reference region for normalisation.
Brain areas reaching the significance threshold were identified in terms of voxel coordinates at the peak significance level and labelled according to Talairach and Tournoux,29 after conversion from SPM space (ie, Montreal Neurological Institute space), using the linear transformations of Brett (www.mrc‐cbu.cam.ac.uk/Imaging). Cluster sizes and locations were recorded for each region in which the voxel‐level corrected significance threshold of p<0.05 was reached, using a height threshold of p = 0.005 (table 2). Anatomical regions were determined by comparing cluster location with a probabilistic atlas.30
Table 2 Brain areas with significantly different perfusion in the group of patients with MCI who were diagnosed with probable Alzheimer's disease on follow‐up (the converters) compared with the normal, stable or decliner groups.
| Extent (voxels) | z Score | Coordinates | Region | BA | |
|---|---|---|---|---|---|
| Normal v converters | |||||
| Converter decreases | 181 | 4.25 | −14, 32, 24 | Lt and Rt ant cingulate (caudal) | 32/24 |
| 80 | 4.19 | −10, −22, 38 | Lt post cingulate | 31 | |
| 296 | 4.13 | 36, 2, −6 | Rt insula | 13 | |
| 128 | 4.14 | 16, 16, 18 | Rt caudate | ||
| 355 | 3.62 | −6, −4, 4 | Thalami | ||
| 111 | 3.37 | 10, −22, 40 | Rt post cingulate | 31 | |
| Converter increases | None | ||||
| Stable v converters | |||||
| Converter decreases | 281 | 4.55 | −10, 34, 20 | Lt and Rt ant cingulate (caudal) | 32 |
| 3.41 | 10, 18, 34 | ||||
| 715 | 4.31 | 40, 0, −8 | Rt insula | 21 | |
| 709 | 4.21 | 14, −34, 10 | Thalami | ||
| 100 | 4.18 | 16, 16, 18 | Rt caudate | ||
| 272 | 4.14 | −38, −28, 8 | Lt insula/temporal | 41 | |
| 163 | 4.11 | 12, −20, 42 | Rt post cingulate | 31 | |
| 137 | 3.50 | −12, −28, 38 | Lt post cingulate | 31 | |
| 119 | 3.26 | 50, 22, 34 | Rt mid frontal | 9 | |
| 105 | 3.16 | −42, 2, −4 | Lt insula | 13 | |
| Converter increases | 571 | 3.68 | −12, 42, −6 | Lt ant cingulate (rostral)/medial frontal | 32 |
| 10 | |||||
| 852 | 3.52 | 6, 32, −8 | Rt ant cingulate (rostral) | 32 | |
| Decliners v converters | |||||
| Converter decreases | 387 | 4.80 | −10, 32, 22 | Lt ant cingulate (caudal) | 32 |
| 1473 | 4.48 | 46, 14, −14 | Rt sup temporal | 38 | |
| 1288 | 4.43 | 14, −34, 10 | Rt thalamus | ||
| 219 | 4.13 | −38, 20, 34 | Lt mid frontal | 9 | |
| 395 | 4.03 | 12, −28, 40 | Rt post cingulate | 31 | |
| 941 | 3.98 | −44, 0, −4 | Lt sup temporal | 22 | |
| 189 | 3.79 | 48, 22, 40 | Rt mid frontal | 8 | |
| 117 | 3.40 | −6, 64, 24 | Lt sup frontal | 10 | |
| Converter increases | 1128 | 4.03 | −26, 40, −8 | Lt mid frontal/ant cingulate (rostral) | 11 |
| 838 | 3.59 | 8, 34, −12 | Rt ant cingulate (rostral)/mid frontal | 32 |
Ant, anterior; BA, Brodmann area; Lt, left; mid, middle; Rt, right; sup, superior.
Significance for each region was corrected for multiple comparisons at the voxel level (p<0.05). Relative decreases and increases are listed for each pairwise comparison. Each region of significant difference is listed with the corresponding cluster size (extent in voxels), the peak voxel z score, the peak voxel coordinates, the anatomical label, and the nearest BA.
In addition, each subject's global mean activity was normalised by cerebellar activity to provide a measure of relative global activity, in order to examine group differences in global mean uptake, which could confound group differences in globally normalised data. An ANOVA was then performed comparing the five groups on this measure.
ROI‐based analyses
A selected set of SPECT ROIs were examined to determine the degree to which the regionally defined perfusion values could be used to discriminate the decliners and converters and to examine the relationship of regional perfusion with neuropsychological performance. The ROIs included: (1) the caudal anterior cingulate, (2) the rostral anterior cingulate and (3) the posterior cingulate. These ROIs were chosen as they reflected the regions with the most consistent decreases and/or increases in perfusion in the pairwise analyses described above. Measures of perfusion were calculated within each region, using the ROI toolbox, and normalised to the mean global perfusion value.
To calculate the classification accuracy for distinguishing decliners from converters, receiver‐operating characteristic curves were examined for each of the three ROIs. The threshold that best discriminated the two groups was selected, based on visual inspection. The sensitivity of this measure was calculated as the number of converters that fell below the threshold, divided by the total number of converters. The specificity of the measure was the number of decliners that fell above the threshold, divided by the total number of decliners.
To examine the relationship between cognitive performance and regional SPECT perfusion, the normalised perfusion values within each of the ROIs were correlated with the neuropsychological measures outlined below.
Neuropsychological tests
The participants in the longitudinal study (groups 1–4) were administered a set of neuropsychological tests as part of their baseline evaluation, as noted above. This battery consisted of 23 neuropsychological measures, including tests of memory, language, spatial ability, attention, executive function and general knowledge.
To determine whether the SPECT perfusion measures were correlated with measures of specific cognitive abilities, we examined the relationship between the ROI perfusion measures described above in 3 of the 23 neuropsychological test measures that had previously been shown to predict which subjects with mild impairments would progress to a diagnosis of Alzheimer's disease within 3 years.16 The cognitive measures selected were: (1) the California Verbal Learning Test,31 (2) the Self‐Ordering Test32 and (3) the Trailmaking Test (TMT), Part B.33 The use of a small set of neuropsychological measures allowed us to reduce the number of statistical comparisons.
Results
SPM analyses
Four‐group comparisons
A one‐way ANOVA of four groups (normal v stable v decliner v converter) showed a significant group effect (F = 88.21; p<0.001). The primary analyses concerned a comparison of the subjects who were diagnosed with probable Alzheimer's disease on follow‐up (the converter group). Table 2 shows that they were significantly different from each of the comparison groups and can be summarised as follows:
Converters versus normals: Perfusion was reduced in converters compared with normals in the right and left caudal anterior cingulate (BA 32/24), the right and left posterior cingulate (BA 31), the right insula (BA 13) and adjacent superior temporal gyrus, and the caudate nucleus and thalamus. No statistically significant perfusion increases were found.
Converters versus stables: Perfusion was reduced in converters compared with the stable subjects in the left and right caudal anterior cingulate (BA 32), left and right insula, and adjacent superior temporal gyrus (BA 13/21/41), thalami, right caudate nucleus, left and right posterior cingulate (BA 31), and in a right prefrontal region (BA 9). Perfusion was, however, increased in converters compared with stable subjects in the left and right rostral portions of the anterior cingulate (BA 32) and the adjacent medial frontal lobe (BA 10).
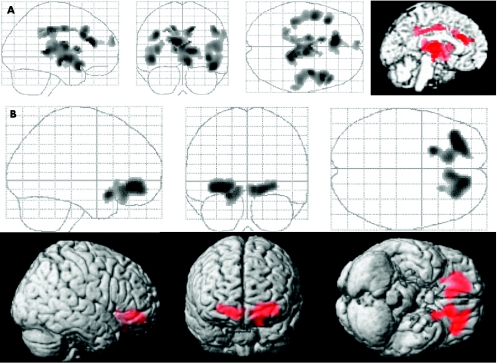
Converters versus decliners: Perfusion was reduced in converters compared with decliners in the left caudal anterior cingulate (BA 32), bilateral superior temporal gyrus (BA 22/38) and adjacent insular regions, and in the bilateral prefrontal regions (BA 8/9/10) and thalamus (fig 1A). Perfusion was increased in converters compared with decliners in the rostral portion of the anterior cingulate (BA 32) and in an adjacent portion of the left inferior frontal lobe (BA 11; fig 1B).
Figure 1 Results of statistical parametric mapping analyses (maximum intensity projections) comparing the patients with mild cognitive impairment (MCI) who progressed to a diagnosis of probable Alzheimer's disease during the follow‐up interval (the converters) with the patients with MCI who declined but did not receive a clinical diagnosis of Alzheimer's disease on follow‐up (the decliners). (A) The brain regions in which the converter group had reduced perfusion compared with the decliner group. (B) The brain regions in which the converter group had increased perfusion compared with the decliner group. Table 2 provides Talairach coordinates of all findings (p<0.05).
Secondary analyses of perfusion decreases compared the normal group with the stable and decliner groups. Decliners had reduced perfusion compared with normals in a region of the right posterior cingulate. The normals differed in perfusion from the decliners in a region of the right posterior cingulate that reached significance at the cluster level (p<0.02; x, y, z = 12, −46, 36; z = 4.46; BA 31). The normal group did not, however, differ from the stable group.
Secondary analyses of perfusion increases compared the normal group with the converter group. Converters had increased perfusion compared with normals in the anterior cingulate and inferior frontal regions (centred at x, y, z = −12, 42, −6; z = 3.46; BA 32/10) that reached significance at the cluster level (p<0.04).
Five‐group comparisons
A one‐way ANOVA of all five groups showed a significant group effect (F = 97.58; p<0.001). Post hoc a priori pairwise comparisons were then performed. The patients with Alzheimer's disease differed significantly from each of the comparison groups, and the regional distribution of these differences can be summarised as follows (table 3):
Table 3 Brain areas with significantly different perfusion in the Alzheimer's disease group compared with the normal, stable, decliner or converter groups.
| Extent (voxels) | z Score | Coordinates | Region | BA | |
|---|---|---|---|---|---|
| Normal v AD | |||||
| AD decreases | 6629 | 5.20 | −42, −62, 38 | Lt inf parietal | 39/40 |
| 862 | 4.87 | 12, −38, 35 | Rt post cingulate | 31 | |
| 105 | 4.46 | −12, 16, 10 | Lt caudate | ||
| 457 | 4.40 | −6, 18, 32 | Lt ant cingulate (caudal) | 32 | |
| 2117 | 4.01 | 48, −54, 18 | Rt sup temporal | 21/22 | |
| 214 | 3.59 | −38, 14, −14 | Lt inf frontal | 47 | |
| 175 | 3.41 | 38, 16, −24 | Rt sup temporal | 38 | |
| 201 | 3.34 | 0, −64, 48 | Parietal precuneus | 7 | |
| 271 | 3.29 | 60, −36, −8 | Rt mid temporal | 21 | |
| AD increases | 6156 | 4.95 | 2, −34, −28 | Rt striatum | |
| Rt thalamus | |||||
| Dorsal midbrain | |||||
| Pons/cerebellum | |||||
| 1184 | 4.42 | −24, −14, 10 | Lt striatum | ||
| Lt thalamus | |||||
| Stable v AD | |||||
| AD decreases | 13017 | 5.83 | −44, −60, 36 | Lt temporoarietal | 39/4021/22 |
| 2224 | 5.23 | 0, 58, 18 | Med frontal, ant cingulate (caudal) | 10/32 | |
| 6525 | 5.19 | 50, −58, 20 | Rt temporoarietal | 7/39/40/21/22 | |
| 2135 | 5.02 | −2, −66, 48 | Lt parietal, post cingulate | 7/31 | |
| 570 | 4.50 | 48, 22, 26 | Rt frontal | 46 | |
| 1808 | 4.33 | 30, 14, −30 | Rt sup temporal | 38 | |
| AD increases | 6486 | 5.82 | 20, −14, 10 | Rt striatum thalamus | |
| Dorsal midbrain | |||||
| Pons/cerebellum | |||||
| Rt medial frontal | 6 | ||||
| 2376 | 4.96 | −20, −10, 12 | Lt thalamus | ||
| Lt striatum | |||||
| Lt lat frontal | 6 | ||||
| Decliners v AD | |||||
| AD decreases | 15487 | 6.06 | −44, −60, 38 | Lt temporoparietal | 39/40 |
| 21/22 | |||||
| 2467 | 5.76 | −2, 60, 20 | Lt/Rt med frontal, Lt/Rt ant cingulate (caudal) | 10/32 | |
| 2365 | 5.73 | 12, −34, 38 | Lt/Rt post cingulate | 7/31 | |
| 6335 | 5.11 | 52, −20, 18 | Rt temporoparietal | 39/40 | |
| 21/22 | |||||
| AD increases | 10039 | 6.19 | 2, −34, −18 | Rt and Lt striatum | |
| Thalamus | |||||
| Dorsal midbrain | |||||
| Pons/cerebellum | |||||
| Rt med frontal | 6 | ||||
| 2283 | 5.07 | −18, −12, 12 | Lt striatum | ||
| 826 | 4.49 | −14, −14, 60 | Lt lat sup frontal | 6 | |
| Converter v AD | |||||
| AD decreases | 3843 | 5.20 | −54, −54, 10 | Lt parietal, temporal | 39/40 |
| 21/22 | |||||
| 3813 | 5.06 | 34, −20, −24 | Rt parahippocampal temporal parietal | 36/21 | |
| 39/40 | |||||
| 867 | 4.16 | 4, −48, 40 | Rt post cingulate | 31 | |
| AD increases | |||||
| 3851 | 4.84 | 6, −28, 0 | Rt striatum | ||
| Rt thalamus | |||||
| Dorsal midbrain | |||||
| Pons/cerebellum | |||||
| 634 | 3.29 | −18, −10, 10 | Lt striatum | ||
| Rt thalamus |
AD, Alzheimer's disease; ant, anterior; BA, Brodmann area; inf, inferior; Lt, left; med, medial; Rt, right; sup, superior.
Significance for each region was corrected for multiple comparisons at the voxel level (p<0.05). Relative decreases and increases are listed for each pairwise comparison. Each region of significant difference is listed with the corresponding cluster size (extent in voxels), the peak voxel z score, the peak voxel coordinates, the anatomical label, and the nearest BA.
Patients with Alzheimer's disease versus normals: Perfusion was reduced in patients with Alzheimer's disease compared with normals in large bilateral temporoparietal, posterior cingulate and precuneus regions (BA 7/31/39/40/21/22/23), the left anterior cingulate (BA 32), the left caudate nucleus and the left inferior frontal (BA 47). Perfusion was increased in patients with Alzheimer's disease compared with normals in a portion of the thalami, dorsal midbrain, pons and cerebellum, and in a bilateral region of the ventral striatum.
Patients with Alzheimer's disease versus stables: Perfusion was reduced in patients with Alzheimer's disease compared with the stable group in large bilateral temporoparietal, medial frontal and caudal anterior cingulate regions, and in the left posterior cingulate and right superior temporal regions. Perfusion was increased in patients with Alzheimer's disease compared with decliners in the thalami, dorsal midbrain, pons and cerebellum, in a bilateral striatal region, and in a bilateral frontal region.
Patients with Alzheimer's disease versus decliners: Perfusion was reduced in patients with Alzheimer's disease compared with decliners in large bilateral temporoparietal, medial frontal, caudal anterior cingulate and posterior cingulate regions. Perfusion was increased in patients with Alzheimer's disease compared with decliners in the thalami, dorsal midbrain, pons and cerebellum, in the bilateral striatum, and in a bilateral frontal region.
Patients with Alzheimer's disease versus converters: Perfusion was reduced in patients with Alzheimer's disease versus converters in the right posterior cingulate (BA 31) and in a large, bilateral temporoparietal region (BA 21/22/36/39/40; table 2). Perfusion was increased in patients with Alzheimer's disease compared with converters in bilateral striatal, thalamic, midbrain and brain stem regions (table 3).
Effect of global normalisation on group differences
When the voxel‐based data were normalised to the cerebellar ROI, the results of the one‐way ANOVAs (for both the four‐group and the five‐group comparisons) as well as the pairwise group comparisons were similar (data not shown). The mean relative global perfusion for each of the five groups was also compared, using ANOVA. This analysis was not significant (p>0.10) for either the four‐group analyses or the five‐group analyses.
Correlations with neuropsychological test scores
Three ROIs were selected for the correlational analysis. The caudal anterior cingulate and the posterior cingulate were selected because these were consistently decreased in perfusion in comparison of the converters with the other groups, and because these regions have been reported previously to be reduced in prodromal Alzheimer's disease. The rostral anterior cingulate was selected because it was the ROI with the most consistent increase in perfusion in comparison of the converters with the other groups. The correlations between these measures and each of the three neuropsychological test scores mentioned above (California Verbal Learning Test, Self‐Ordering Test and TMT) were then examined, using data from groups 1–4.
Higher perfusion in the rostral anterior cingulate and adjacent inferior frontal regions was associated with poorer performance (ie, higher score, positive t value) on the TMT (p<0.05). Lower perfusion in the caudal AC was associated with poorer performance (ie, lower scores, negative t value) on the TMT (p<0.006). No significant associations were seen between the posterior cingulate and any of the three neuropsychological tests.
Group classification using ROIs
The perfusion within the posterior cingulate ROI discriminated decliners from converters with 79% sensitivity and 67% specificity; this reflected an average decrease in perfusion of approximately 8%. The perfusion within the rostral anterior cingulate discriminated the decliners from converters with 60% sensitivity and 42% specificity; this reflected an average increase in perfusion of approximately 5%.
Discussion
These findings indicate that patients with MCI who are subsequently diagnosed with Alzheimer's disease (ie, the converter group) show significant differences in SPECT brain perfusion from patients with MCI who remain stable or who decline but do not progress to a diagnosis of Alzheimer's disease within a 5‐year follow‐up period. The regions showing the most consistent decreases in comparison of the converters with the other groups were the caudal portion of the anterior cingulate and the posterior cingulate. These findings are consistent with previous reports in both PET6,10,12 and SPECT.13,14
In addition, we identified a region of the inferior and medial frontal lobes that included the rostral anterior cingulate, which had relatively increased perfusion at baseline in subjects who progressed to meet the criteria for Alzheimer's disease compared with the stable and decliner groups. A finding of increased resting perfusion among individuals who subsequently progress to a diagnosis of Alzheimer's disease is similar to the recent report by Huang et al.14 They reported a region of increased perfusion in the inferolateral frontal lobes (BA 45/47) in patients with MCI (mean age 63.4 years) who progressed to a diagnosis of early‐onset Alzheimer's disease within 2 years of evaluation, and who had a mean MMSE score of 25.7. The patients with MCI in our study were older (mean age 75.0 years) and more mildly impaired at baseline (mean MMSE 28.7 years) than the subjects in the Huang et al study.14 This difference in subject population may explain the increased perfusion seen in the medial frontal (BA 11) and anterior cingulate regions (BA 32). As in the Huang et al study, we used more than one normalisation procedure to examine the robustness of our finding. Relative increases in these regions were found, regardless of the normalisation procedure used. No differences in relative global perfusion were found across the groups, suggesting that global differences could not explain the finding of selective increases in brain perfusion. Moreover, higher perfusion was associated with poorer cognitive test performance.
Taken together, these findings suggest that increases in perfusion, as well as decreases, can be observed during the prodromal phase of Alzheimer's disease. Such increases may be a direct result of the pathology associated with Alzheimer's disease—that is, a response by neurones to the accumulation of neuritic plaques and neurofibrillary tangles34 or a response to increased aberrant sprouting of cholinergic synapses.35 An alternative explanation is that such increases may reflect a compensatory response to the increased effort required to perform daily activities.36,37 As we have found increased perfusion during the resting state, the former possibility seems more likely. Factors unrelated to the pathology of Alzheimer's disease may also be involved, including vascular pathology.
In this context, it should be noted that a finding of increased resting perfusion among individuals at risk for Alzheimer's disease is similar to recent results in which activation tasks have been used with both PET and functional MRI. A recent PET study of patients without dementia with Down's syndrome showed increased PET activation in inferior temporal regions during an attention task.38 Likewise, functional MRI studies of patients without dementia at risk for Alzheimer's disease by virtue of mild cognitive deficits39 or genetic factors36 report medial temporal lobe regions of increased activation, using memory tasks.
Comparison of the patients with Alzheimer's disease with each of the groups showed significant reductions in perfusion (table 3), most consistently in large temporoparietal regions, in agreement with previous reports. Increases in perfusion among patients with Alzheimer's disease were found in subcortical regions (eg, striatum and thalami). These findings suggest that, over time, the pattern of perfusion abnormalities evolves, with the evolution of pathology.
When reduced perfusion or metabolism coexists with underlying brain atrophy (as in prodromal or established Alzheimer's disease), both perfusion and atrophy contribute to the reduced activity. It is estimated that the “partial volume” effect related to atrophy likely accounts for 10–15% of the reduction observed in the relatively large regions evaluated in our study.13,40,41 Given the similarity in both global and relative perfusion among the groups in the present study, the finding of selective increases in perfusion is unlikely to be due to underlying brain atrophy or due to decreases in the overall perfusion of the brain.
We have previously analysed SPECT data from many of the same patients with an MRI‐based ROI analysis, rather than SPM, and have found reduced perfusion in a number of medial temporal lobe (MTL) regions in addition to those reported here.13 These findings are similar to those of investigators using a similar approach with PET.9,15 As pointed out by these investigators, the difference in results is likely related to the spatial transformation and heavy smoothing used by SPM. Moreover, functional imaging methods, such as PET and SPECT, primarily assess the synaptic activity of MTL efferent projections. Both factors make it less likely that SPM will identify small MTL regions such as the entorhinal cortex (which shows the pathology of Alzheimer's disease early in the course of Alzheimer's disease), and more likely that it will identify larger cortical regions such as the posterior cingulate (which receives direct projections from the entorhinal cortex). The ROI method that we used previously is, however, labour intensive, requiring manually drawn ROIs that are superimposed on the SPECT images. This makes it more difficult to identify group differences without an a priori hypothesis. The current study identified one such unexpected group difference related to increased rather than decreased perfusion.
Acknowledgements
We thank Dr Mary Hyde for assistance with data management and analysis.
Abbreviations
ANOVA - analysis of variance
CDR - clinical dementia rating
CDR‐SB - CDR Sum of Boxes
MCI - mild cognitive impairment
MMSE - Mini‐Mental State Examination
MRI - magnetic resonance imaging
MTL - medial temporal lobe
PET - positron emission tomography
ROI - regions of interest
SPECT - single photon emission computed tomography
SPM - statistical parametric mapping
TMT - Trailmaking Test
Footnotes
Funding: This research was supported by grants from the NIA (PO1‐AG04953) and the Alzheimer Association (# IRG‐99‐1515). This research was approved by the Massachusetts General Hospital Human Research Committee (1999‐P‐005425).
Competing interests: None declared.
References
- 1.Jagust W, Budinger T, Reed B. The diagnosis of dementia with single photon emission computed tomography. Arch Neurol 198744258–262. [DOI] [PubMed] [Google Scholar]
- 2.Bradley K M, O'Sullivan V T, Soper N D.et al Cerebral perfusion SPET correlated with Braak pathological stage in Alzheimer's disease. Brain 20021251772–1781. [DOI] [PubMed] [Google Scholar]
- 3.Johnson K A, Jones K, Holman B L.et al Preclinical prediction of Alzheimer's disease using SPECT. Neurology 1998501563–1571. [DOI] [PubMed] [Google Scholar]
- 4.Kogure D, Matsuda H, Ohnishi T.et al Longitudinal evaluation of early Alzheimer's disease using brain perfusion SPECT. J Nucl Med 2000411155–1162. [PubMed] [Google Scholar]
- 5.Herholz K, Salmon E, Perani D.et al Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage 200217302–316. [DOI] [PubMed] [Google Scholar]
- 6.Minoshima S, Giordani B, Berent S.et al Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol 19974285–94. [DOI] [PubMed] [Google Scholar]
- 7.Okamura N, Shinkawa M, Arai H.et al Prediction of progression in patients with mild cognitive impairment using IMP‐SPECT. Nippon Ronen Igakkai Zasshi 200037974–978. [DOI] [PubMed] [Google Scholar]
- 8.Arnaiz E, Jelic V, Almkvist O.et al Impaired cerebral glucose metabolism and cognitive functioning predict deterioration in mild cognitive impairment. Neuroreport 200112851–855. [DOI] [PubMed] [Google Scholar]
- 9.De Santi S, de Leon M, Rusineck H.et al Hippocampal formation, glucose metabolism and volume loss in MCI and AD. Neurobiol Aging 200122529–539. [DOI] [PubMed] [Google Scholar]
- 10.Jagust W J, Eberling J L, Wu C C.et al Brain function and cognition in a community sample of elderly Latinos. Neurology 200259378–383. [DOI] [PubMed] [Google Scholar]
- 11.Chetelat G, Desgranges B, de la Sayette V.et al Mild cognitive impairment: can FDG‐PET predict who is to rapidly convert to Alzheimer's disease? Neurology 2003601374–1377. [DOI] [PubMed] [Google Scholar]
- 12.Drzezga A, Lautenschlager N, Siebner H.et al Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow‐up study. Eur J Nucl Med Mol Imaging 2003301104–1113. [DOI] [PubMed] [Google Scholar]
- 13.El Fakhri G, Kijewski M F, Johnson K A.et al MRI‐guided SPECT perfusion measures and volumetric MRI in prodromal Alzheimer disease. Arch Neurol 2003601066–1072. [DOI] [PubMed] [Google Scholar]
- 14.Huang C, Wahland L ‐ O, Almkvist O.et al Voxel‐ and VOI‐based analysis of SPECT CBF in relation to clinical and psychological heterogeneity of mild cognitive impairment. NeuroImage 2003191137–1144. [DOI] [PubMed] [Google Scholar]
- 15.Nestor P J, Fryer T D, Smielewski P.et al Limbic hypometabolism in Alzheimer's disease and mild cognitive impairment. Ann Neurol 200354343–351. [DOI] [PubMed] [Google Scholar]
- 16.Albert M, Moss M, Tanzi R.et al Preclinical prediction of AD using neuropsychological tests. J Intl Neuropsychol Soc 20017631–639. [DOI] [PubMed] [Google Scholar]
- 17.Morris J C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993432412–2414. [DOI] [PubMed] [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M F.et al Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Workgroup under the auspices of Department of Health and Human Services Task Force. Neurology 198434939–944. [DOI] [PubMed] [Google Scholar]
- 19.Hachinski V. Cerebral blood flow differentiation of Alzheimer's disease from multi‐infarct dementia. In: Katzman R, Terry R, Bick K, eds. Alzheimer's disease: senile dementia and related disorders. New York: Raven Press, 197897–103.
- 20.Daly E, Zaitchik D, Copeland M.et al Predicting ‘conversion' to AD using standardized clinical information. Arch Neurol 200057675–680. [DOI] [PubMed] [Google Scholar]
- 21.Folstein M, Folstein S, McHugh P J. “Mini‐Mental State”. A practical method for grading the cognitive state of patients for the clinician. Psychiatr Res 197512189–198. [DOI] [PubMed] [Google Scholar]
- 22.Davis H, Rockwood K. Conceptualization of mild cognitive impairment: a review. Int J Geriatr Psychiatry 200419313–319. [DOI] [PubMed] [Google Scholar]
- 23.Petersen R, Thomas R, Grundman M.et al Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 20053522379–2388. [DOI] [PubMed] [Google Scholar]
- 24.Petersen R. Mild cognitive impairment. J Intern Med 2004256183–194. [DOI] [PubMed] [Google Scholar]
- 25.Holman B, Carvalho P, Zimmerman R.et al Brain perfusion SPECT using an annular single crystal camera: initial clinical experience. J Nucl Med 1990311456–1561. [PubMed] [Google Scholar]
- 26.Friston K J, Holmes A P, Worsley K J.et al Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapping 19952189–210. [Google Scholar]
- 27.Brett M, Anton J ‐ L, Valabregue R.et al Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conference on Functional Mapping of the Human Brain 2–6 June, 2002, Sendai, Japan. NeuroImage. 2002;16: 497 (available on CD‐ROM),
- 28.Genovese C R, Lazar N A, Nichols T E. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 200215870–878. [DOI] [PubMed] [Google Scholar]
- 29.Talairach J, Tournoux P.Co‐planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers, 1988
- 30.Lancaster J L, Woldorff M G, Parsons L M.et al Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapping 200010120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delis D, Kramer J, Kaplan E.et alThe California Verbal Learning Test. New York: Psychological Corp, 1987
- 32.Petrides M, Milner B. Deficits in subject‐ordered tasks after frontal and temporal lobe lesions in man. Neuropsychologia 198220249–262. [DOI] [PubMed] [Google Scholar]
- 33.Reitan R M. Validity of the Trailmaking Test as an indicator of organic brain damage. Percept Mot Skills 19588271–276. [Google Scholar]
- 34.Mesulam M M. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron 199924521–529. [DOI] [PubMed] [Google Scholar]
- 35.Masliah E, Alford M, Adame A.et al Abeta1‐42 promotes cholinergic sprouting in patients with AD and Lewy body variant of AD. Neurology 200361206–211. [DOI] [PubMed] [Google Scholar]
- 36.Bookheimer S Y, Strojwas M H, Cohen M S.et al Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med 2000343450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grady C, McIntosh A R, Beig S.et al Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer's disease. Neuroscience 200323986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haier R, Alkire M, White N.et al Temporal cortex hypermetabolism in Down syndrome prior to the onset of dementia. Neurology 2003611673–1679. [DOI] [PubMed] [Google Scholar]
- 39.Dickerson B, Salat D, Bates J.et al MRI measures of medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol 20045627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meltzer C, Zubieta J, Brandt J.et al Regional hypometabolism in Alzheimer's disease as measured by positron emission tomography after correction for effects of partial volume averaging. Neurology 199647454–461. [DOI] [PubMed] [Google Scholar]
- 41.de Leon M, Convit A, Wolf O.et al Prediction of cognitive decline in normal elderly subjects with 2‐[(18)F] fluoro‐2‐deoxy‐D‐glucose/positron‐emission tomography (FDG/PET). Proc Natl Acad Sci USA 20019810966–10971. [DOI] [PMC free article] [PubMed] [Google Scholar]