Abstract
The role of amyloid metabolism in the pathophysiology of frontotemporal lobar degeneration (FTLD) has yet to be elucidated. We compared CSF levels of amyloid beta 1–40 (Aβ40) and amyloid beta 1–42 (Aβ42) in patients with FTLD (n = 21) versus patients with Alzheimer's disease (AD, n = 39) and in control subjects (n = 30). While in AD cases Aβ42 levels were lower and CSF Aβ40 levels equal to those in controls, a significant decrease in Aβ40 and increase in the CSF Aβ42/Aβ40 ratio was observed in FTLD compared with AD and control subjects. These findings favour a differential involvement of amyloid β peptides in FTLD compared with AD.
Frontotemporal lobar degeneration (FTLD) is a spectrum of neurodegenerative disorders affecting the frontal and/or temporal lobes, clinically characterised by behaviour and/or language disturbances.1 Pathological findings in FTLD can be classified into five groups.2 As distinct pathological processes in the brain may result in specific CSF biomarker profiles, the highly varying levels of CSF biomarkers in FTLD might reflect the pathological heterogeneity of FTLD.
Alzheimer's disease (AD) is the most common cause of dementia and is pathologically characterised by the combination of plaques, mainly consisting of amyloid beta (1–42) (Aβ42) and to a lesser extent amyloid beta (1–40) (Aβ40), as well as tau positive neurofibrillary tangles. In AD, Aβ42 levels in CSF have been consistently found to be decreased3 whereas levels of the more soluble CSF Aβ40 are normal.4 Although intracerebral deposition of Aβ is not a hallmark of FTLD, CSF levels of Aβ42 can be moderately decreased in FTLD.3 These data suggest that the presence or absence of intracerebral Aβ deposition is not the only determinant of CSF Aβ levels in AD and FTLD. As relatively little is known about CSF Aβ40 levels in FTLD, we aimed to determine levels of CSF Aβ42 and Aβ40 in FTLD, AD and in control cases.
Methods
Patients
Twenty‐one patients with FTLD, 39 patients with probable AD and 30 cognitively healthy controls were recruited from the Alzheimer Centre, VU University Medical Centre, Amsterdam, the Netherlands. The clinical diagnoses were made in conference by a multidisciplinary team based on accepted clinical diagnostic criteria.1,5 During the diagnostic procedure, all patients underwent screening laboratory tests, psychometric tests, EEG and MRI of the brain. In four patients with FTLD with normal or non‐conclusive structural neuroimaging findings, 99mhexamethylpropyleneamine single photon emission computed tomography was performed. One patient with FTLD had a family history suggestive of autosomal dominant presenile dementia but refused genetic investigation. The other cases were not considered for mutation screening. All patients were living in the community. The control group consisted of 20 subjects with subjective memory complaints but no abnormalities on diagnostic screening, five cognitively healthy spouses of patients, three subjects with no complaints but a positive family history of dementia and two subjects with other neurological conditions but no cognitive symptoms. The Mini Mental State Examination was performed in 20/21 FTLD patients, all AD patients and 29/30 control subjects. The Clinical Dementia Rating was used as a measure of dementia severity. Subjects were only included in the study if the diagnosis did not change after 1 year of follow‐up, to compensate for the lack of histopathological verification. Disease duration was defined as the time difference between disease onset, based on history, and time of lumbar puncture. All subjects gave written informed consent to participate in the study. Approval was given by the Ethical Review Board of the VU University Medical Centre.
Laboratory analysis
CSF was obtained by lumbar puncture between the L3/L4 or L4/L5 intervertebral space, and 12 ml were collected in polypropylene tubes. Within 1 h, CSF samples were centrifuged at 3000 rpm for 10 min at 4°C. CSF was aliquoted in polypropylene tubes of 0.5 or 1 ml, and stored at −80°C until analysis. CSF Aβ42 and Aβ40 were determined using specific ELISAs, as previously described.6 The monoclonal antibody 6E10 (Signet Labs, Dedham, Massachusetts, USA), which recognises residues 1–16 of Aβ, was used as the capture antibody, and the polyclonal antibodies R208 and R165 (specific for Aβ40 and Aβ42, respectively) as detector antibodies.
Statistical analysis
Clinical variables and levels of CSF markers were compared between the three groups using non‐parametric tests (Kruskal‐Wallis followed by the Mann‐Whitney U test). A p value of <0.05 was considered to reflect statistical significance.
Results
CSF biomarkers in FTLD, AD and controls
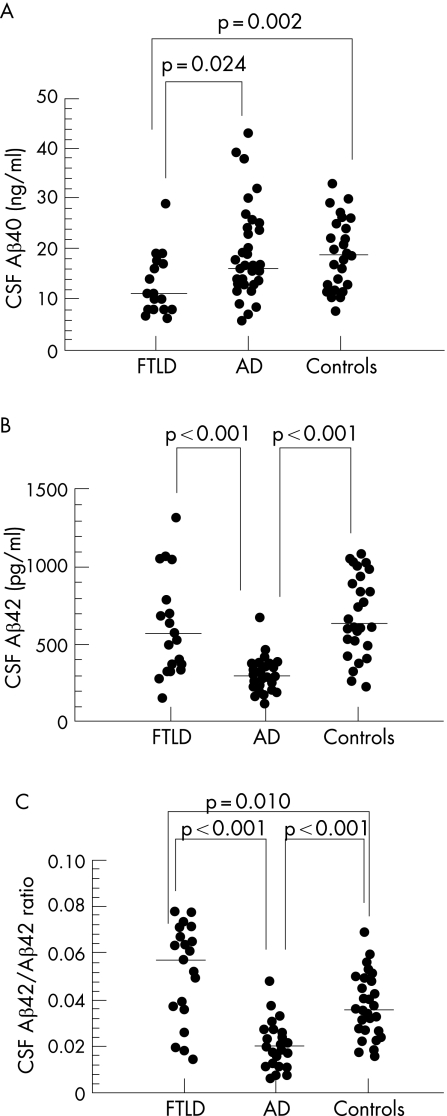
Clinical variables and CSF Aβ levels are shown in table 1. The median level of CSF Aβ40 was significantly lower in FTLD compared with AD and control subjects. Because Aβ42 levels were decreased in AD, but not in FTLD patients, compared with controls, the ratio of CSF Aβ42 to Aβ40 was significantly increased in FTLD compared with the two other groups.
Table 1 Clinical variables and CSF amyloid beta levels.
| FTLD (n = 21) | AD (n = 39) | Controls (n = 30) | p Values | |
|---|---|---|---|---|
| Age (y) | 63 (52–85) | 62 (52–79) | 64 (32–79) | 0.38 |
| Disease duration (y) | 3 (1–11) | 4 (1–11) | – | 0.05 |
| CDR | 1 (1–2) | 1 (1–3) | – | 0.18 |
| MMSE | 24 (3–29) | 20 (3–28) | 30 (25–30) | 0.50* |
| <0.001† | ||||
| <0.001‡ | ||||
| CSF Aβ40 (ng/ml) | 11 (6–29) | 16 (6–43) | 19 (8–33) | 0.024* |
| 0.002† | ||||
| 0.46‡ | ||||
| CSF Aβ42 (pg/ml) | 576 (151–1324) | 288 (116–674) | 629 (218–1075) | <0.001* |
| 0.61† | ||||
| <0.001‡ | ||||
| CSF Aβ42/Aβ40 | 0.057 (0.014–0.078) | 0.017 (0.006–0.048) | 0.035 (0.016–0.069) | <0.001* |
| 0.010† | ||||
| <0.001‡ |
Aβ40, amyloid beta 1–40; Aβ42, amyloid beta 1–42; AD, Alzheimer's disease; CDR, Clinical Dementia Rating; FTLD, frontotemporal lobar degeneration; MMSE, Mini Mental State Examination; y, years.
Significant differences are displayed in bold: *difference between FTLD and AD; †difference between FTLD and control subjects; ‡difference between AD and control subjects.
Values are medians (range).
The CSF Aβ42/Aβ40 ratio was significantly lower in AD compared with control subjects. Individual values of CSF Aβ40, Aβ42 and their ratio are shown in fig 1.
Figure 1 (A–C) Scatterplots of individual levels of CSF amyloid beta 1–40 (Aβ40) (A), amyloid beta 1–42 (Aβ42) (B) and the Aβ42/Aβ40 ratio in patients with frontotemporal lobar degeneration (FTLD) and Alzheimer's disease (AD) and in controls. Significant differences are indicated. Horizontal lines represent median values.
Discussion
We found that the median level of CSF Aβ40 was decreased and the CSF Aβ42/Aβ40 ratio increased in FTLD compared with AD and cognitively healthy controls. Our results are supported by only one study, which found decreased CSF Aβ40 levels in two of five patients with FTLD.7 In another study, a linear correlation between the CSF levels of Aβ40 and the degree of frontal atrophy on MRI was observed in patients with FTLD, suggesting higher CSF Aβ40 levels in FTLD patients with more frontal atrophy.8 However, because of the lack of a proper control group, the results of this study cannot be compared with ours.
Our findings shed new light on the role of amyloid metabolism in FTLD. In AD, accumulation of Aβ42 and Aβ40 in parenchymal extracellular plaques are a pathological hallmark. Although the majority of AD cases are sporadic and the most important risk factor in developing AD is age, possible pathophysiological processes can be derived from mutations in three early onset AD genes: the β‐amyloid precursor protein, and the presenilins 1 and 2 (PS‐1 and PS‐2). Mutations in one of these genes leads to increased production of Aβ from its precursor and formation of amyloid plaques. An increased extracellular Aβ42/Aβ40 ratio in vitro and in vivo appears to be a characteristic of the presenilin mutations.9 However, no mismetabolism of amyloid has been demonstrated in sporadic FTLD. There is only one post‐mortem study in FTLD that found Aβ42 containing plaques in a considerable number of patients.10 In this study, Aβ40 containing plaques were only occasionally seen. The finding of plaques was associated with older age and a higher APOE ε4 allele frequency and was considered coincidental.
In hereditary FTLD caused by tau mutations, increased soluble Aβ40 and Aβ42 in the absence of intracerebral amyloid deposits was described in eight patients.11 Abundant amyloid deposition has been described in a FTLD patient with an R406W tau mutation.12 To date, no indications of disturbances of amyloid metabolism have been reported in patients with progranulin mutations. More intriguing is the possible relationship between PS‐1 mutations and FTLD, although in autopsied cases no intracerebral amyloid plaques were found.13,14 The finding of an additional IVSI+IG → A progranulin mutation in a patient with a previously reported insR352 PS‐1 mutation, however, raises the question of whether PS‐1 mutations lacking amyloid histopathology are really causative of FTLD.14 The presence of increased CSF Aβ40 and Aβ42 levels compared with controls in the latter case remains unexplained.15 Thus in at least some hereditary variants of FTLD, disturbances in amyloid metabolism seem to play a role. These abnormalities do not necessarily lead to intracerebral amyloid deposition, but can indirectly be demonstrated by measuring soluble amyloid fractions or CSF amyloid concentrations.
A limitation of our study is the lack of pathological or genetic confirmation of the clinical diagnosis. Also, the relative short disease duration in both disease groups might have contributed to possible misdiagnosis. The diagnosis of early onset dementia may be complex, in particular because atypical presentations of AD have been described.16 However, as the clinical diagnosis was made in conference in a tertiary referral setting and confirmed by a follow‐up period of at least 1 year, we are confident we achieved a high diagnostic accuracy.
Our study is the first to examine levels of CSF Aβ40 in relation to CSF Aβ42 in FTLD patients compared with AD patients and cognitively healthy controls. Our findings suggest that β‐amyloid precursor protein metabolism is either decreased or altered in sporadic FTLD and deserve future study.
Abbreviations
Aβ40 - amyloid beta 1–40
Aβ42 - amyloid beta 1–42
AD - Alzheimer's disease
FTLD - frontotemporal lobar degeneration
PS‐1 - presenilin 1
Footnotes
Competing interests: None.
References
- 1.Neary D, Snowden J S, Gustafson L.et al Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998511546–1554. [DOI] [PubMed] [Google Scholar]
- 2.McKhann G M, Albert M S, Grossman M.et al Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol 2001581803–1809. [DOI] [PubMed] [Google Scholar]
- 3.Blennow K, Hampel H. CSF markers for incipient Alzheimer's disease. Lancet Neurol 20032605–613. [DOI] [PubMed] [Google Scholar]
- 4.Mehta P D, Pirttila T, Mehta S P.et al Plasma and cerebrospinal fluid levels of amyloid beta proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol 200057100–105. [DOI] [PubMed] [Google Scholar]
- 5.McKhann G, Drachman D, Folstein M.et al Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 198434939–944. [DOI] [PubMed] [Google Scholar]
- 6.Mehta P D, Mehta S P, Fedor B.et al Plasma amyloid beta protein 1–42 levels are increased in old Down Syndrome but not in young Down Syndrome. Neurosci Lett 2003342155–158. [DOI] [PubMed] [Google Scholar]
- 7.Jauss M, Herholz K, Kracht L.et al Frontotemporal dementia: clinical, neuroimaging, and molecular biological findings in 6 patients. Eur Arch Psychiatry Clin Neurosci 2001251225–231. [DOI] [PubMed] [Google Scholar]
- 8.Andersen C, Jensen M, Lannfelt L.et al Amyloid Abeta 40 CSF concentrations correlate to frontal lobe atrophy in frontotemporal dementia. Neuroreport 200011287–290. [DOI] [PubMed] [Google Scholar]
- 9.Borchelt D R, Thinakaran G, Eckman C B.et al Familial Alzheimer's disease‐linked presenilin 1 variants elevate Aβ (1–42)/(1–40) in vitro and in vivo. Neuron 1996171005–1012. [DOI] [PubMed] [Google Scholar]
- 10.Mann D M, McDonagh A M, Pickering‐Brown S M.et al Amyloid beta protein deposition in patients with frontotemporal lobar degeneration: relationship to age and apolipoprotein E genotype. Neurosci Lett 2001304161–164. [DOI] [PubMed] [Google Scholar]
- 11.Vitali A, Piccini A, Borghi R.et al Soluble amyloid beta‐protein is increased in frontotemporal dementia with Tau gene mutations. J Alzheimers Dis 2004645–51. [DOI] [PubMed] [Google Scholar]
- 12.Rosso S M, Kamphorst W, Ravid R.et al Coexistent tau and amyloid pathology in hereditary frontotemporal dementia with tau mutations. Ann NY Acad Sci 2000920115–119. [DOI] [PubMed] [Google Scholar]
- 13.Dermaut B, Kumar‐Singh S, Engelborghs S.et al A novel presenilin 1 mutation associated with Pick's disease but not beta‐amyloid plaques. Ann Neurol 200455617–626. [DOI] [PubMed] [Google Scholar]
- 14.Boeve B F, Baker M, Dickson D W.et al Frontotemporal dementia and parkinsonism associated with the IVSI+IG→A mutation in progranulin: a clinicopathological study. Brain 20061293103–3114. [DOI] [PubMed] [Google Scholar]
- 15.Tang‐Wai D, Lewis P, Boeve B.et al Familial frontotemporal dementia associated with a novel presenilin‐1 mutation. Dement Geriatr Cogn Disord 20021413–21. [DOI] [PubMed] [Google Scholar]
- 16.Johnson J K, Head E, Kim R.et al Clinical and pathological evidence for a frontal variant of Alzheimer's disease. Arch Neurol 1999561233–1239. [DOI] [PubMed] [Google Scholar]