Abstract
Objective
To evaluate the effectiveness of a personalised home programme of exercises and strategies for repeat fallers with Parkinson's disease (PD).
Method
Patients with a confirmed diagnosis of idiopathic PD, independently mobile, living at home in the community, experiencing more than one fall in the previous 12 months and with intact gross cognitive function were invited to participate in this randomised controlled trial. Usual care was compared with a personalised 6 week, home based exercise and strategy programme. The primary outcomes were rates of falling at 8 weeks and 6 months. Whether participants had repeat fallen, nearly fallen or experienced injurious falls were also examined. Functional Reach, the Berg Balance Test, PD Self‐assessment Scale and the Euro Quol were rated by a blinded assessor.
Results
Participants were randomised to the exercise (n = 70) and control (n = 72) groups. There was a consistent trend towards lower fall rates in the exercise group at both 8 weeks and 6 months and lower rates of injurious falls needing medical attention at 6 months. Lower rates of repeat near falling were evident for the exercise group at 8 weeks (p = 0.004) and 6 months (p = 0.007). There was a positive effect of exercises at 6 months on Functional Reach (p = 0.009) and quality of life (p = 0.033). No significant differences were found on other secondary outcomes measures.
Conclusion
There was a trend towards a reduction in fall events and injurious falls with a positive effect of exercises on near falls and quality of life.
Postural instability and falls among people with Parkinson's disease (PD) are common. In contrast with the estimated one‐third of the healthy population over 65 years who experience a fall,1 two‐thirds of people living in the community with PD will have fallen in the previous 12 months2 and those who have fallen two or more times in the previous year are likely to fall again in the next 3 months.3 As falls following PD can be injurious,4 prevention is important but postural instability is difficult to treat with medication. Physiotherapy may provide effective treatment for people with PD but two Cochrane reviews in 2001 on the general physical management of people with PD concluded there was insufficient evidence to support or refute the efficacy of physiotherapy or one form of physiotherapy over another for people with PD, and highlighted the need for more randomised controlled trials to test standard physiotherapy.5,6 Most of the trials included in the systematic reviews recruited less than 20 subjects, and the use of poor research design and methodology was a common finding of the reviewers.
Review of the literature on falls management among the general elderly population confirms that multidisciplinary fall prevention programmes can be beneficial for elderly people.7,8 The most effective intervention was a multifactorial fall risk assessment and management programme. Exercise programmes, such as moderate intensity muscle strengthening and balance training, individually prescribed at home by a trained health professional, have been shown to be effective in reducing fall frequency among the elderly population living in the community.7,8,9,10,11
The purpose of this trial was to evaluate the effectiveness of a personalised home based exercise programme (activities selected from a menu of muscle strengthening, stretches, balance retraining and cognitive movement strategies for learning and compensating), administered by a physiotherapist for reducing fall events among people with PD. The research question addressed was: do repeat fallers with PD, who participate in an exercise programme of strength, balance training and strategies, experience fewer falls, near falls or injuries than those who do not?
Methods
The trial was conducted between October 2002 and April 2005.
Recruitment
Participants were identified through the clinical registers of three PD specialists in two NHS trusts in Dorset, UK. In one trust the specialist had 565 patients on his register, in the other two, specialists had 542 patients in the trial area. In total there were 1107 potential subjects. We worked closely with local PD nurses to identify those suitable for invitation to participate and to document reasons for exclusion. The trial eligibility criteria were as follows: confirmed diagnosis of idiopathic PD, independently mobile, living at home in the community, experienced more than one fall in the previous 12 months and passed a screening test12 for gross cognitive impairment. The inclusion criterion of more than one fall in the previous year ensured that trial participants would be at risk of subsequent falling.3 The exclusion criteria were unable to participate in assessments because of pain, and acute medical condition and in receipt of, or soon to receive, treatment. Following approval from the PD nurse to approach a suitable person identified from the register, a letter was sent to them from their consultant asking if they would like to participate in the trial.
Randomisation
Randomisation was stratified by NHS Trust using blocks of size four. After the baseline assessment by the assessor, the treating physiotherapist obtained the random allocation by telephoning the Medical Statistics Group at the University of Southampton, Southampton, UK. Participants were informed of their allocation by telephone. The assessor thus remained blind to the group allocation.
Assessments
The battery of tests was conducted in the participant's home at baseline, and at 8 weeks and 6 months after randomisation by the assessing researcher. We aimed to assess the participants mid‐way between drug doses. The trial definition of a fall was “an event that resulted in a person coming to rest unintentionally on the ground or other lower level, not as a result of a major intrinsic event or overwhelming hazard”.13 A near fall was an occasion on which an individual felt that they were going to fall but did not actually do so.14,15,16 At the screening interview, participants completed the falls screening test14 and were classed as being a repeat faller if they had experienced two or more falls during the past 12 months. Fall events that were experienced during the trial period were recorded prospectively using self‐completed diaries. Each month, participants were sent a falls diary sheet, consisting of daily numbered date boxes. Individuals recorded “F” for a “fall” and “NF” for a “near fall” whenever these occurred, and returned the sheets to the secretary in a stamped addressed envelope. Participants were also asked to record injuries as a result of falls (cuts and bruises, fractures or other trauma) and whether they attended the hospital, sought other forms of medical help or self‐managed their injuries. The primary outcomes were self‐reported falling or not at 8 weeks and 6 months from the falls diary.
At entry to the trial, a medical history was taken, including details of current medications, living status and current rehabilitation input. Disease severity was recorded using the Hoehn and Yahr Scale17 (five point scale) and the motor assessment part of the Unified Parkinson's Disease Rating Scale18 (0–108; low = good) at the start of the trial. At baseline, 8 weeks and 6 months, the following physical measures were completed: the Functional Reach19 (in cm), the Berg Balance Test20 (0–56; high = good) the “timed up and go test”21(s), and the “chair stand test”22 (s). A measure of the impact of disability was recorded using the Self‐assessment Parkinson's Disease Disability Scale23 (SAS) (25–125; low = good) and the Euro Quol EQ‐5D24 quality of life thermometer (0–100; high = good). Participants were questioned at 6 weeks and 6 months about receiving rehabilitation external to the trial.
Intervention
Participants in the experimental group were visited weekly at home by a physiotherapist. Following assessment, treatment goals were established with participants and exercises from the exercise menu were taught by a physiotherapist. The exercise menu was designed with six levels of exercise progression and comprised muscle strengthening (knee and hip extensors, hip abductors), range of movement (ankle, pelvic tilt, trunk and head), balance training (static, dynamic and functional) and walking (inside and outside). Strategies for falls prevention and movement initiation and compensation were taught by the physiotherapist. Exercises were chosen at the appropriate level for each individual and, if possible, progressed at each visit which would last approximately 1 h. For example, standing from sitting was progressed by increasing practice repetition, lowering the height of the chair and progressing to stepping up. Participants were asked to complete the exercises daily and to keep a record of their exercising on a standardised form. Safety was ensured by appropriate prescribing of exercises, giving instructions with illustrations on each exercise and a contact number for the physiotherapist. Records were kept of initial goals and individual treatment plans for all participants in the experimental group. After the initial 6 week treatment period, the intervention group was telephoned each month by the treating researcher (a physiotherapist) in order to encourage participants to continue their exercises and to provide an opportunity to discuss any problems arising. The control group received usual care which, for the vast majority, comprised contact with a local PD nurse. To increase adherence, they were offered advice about exercises from the treating researcher when they reached the end of follow‐up. Participation by individuals in the exercise or control group in rehabilitation external to the trial was monitored.
Sample size and statistical analysis
A sample size of 100 in each group was required for the trial to have 80% power to detect a reduction from 70% to 50% in fall rates.2 The pre‐planned analysis compared fall rates in a logistic regression, controlled for the number of falls reported in the previous year, centre and the SAS (an important predictor of falling in Ashburn et al2). The unadjusted differences in fall rates and associated 95% confidence intervals (CI) were calculated in StatXact 6. Analysis was on an intention to treat basis in that subjects were included in their allocated group irrespective of the number of visits they received or the extent to which they practised their exercises. No subgroup analyses were planned but after the results had been examined, fall rates were compared between the intervention and control groups separately for less and more severe subgroups, defined by having Hoehn and Yahr scores of 2–3 or 4, respectively, and the interaction between the intervention comparison and subgroup was tested in a logistic regression. Other outcomes were compared in either logistic or multiple regression models controlling for the outcome variable measured at baseline (is available), centre and the SAS.
Results
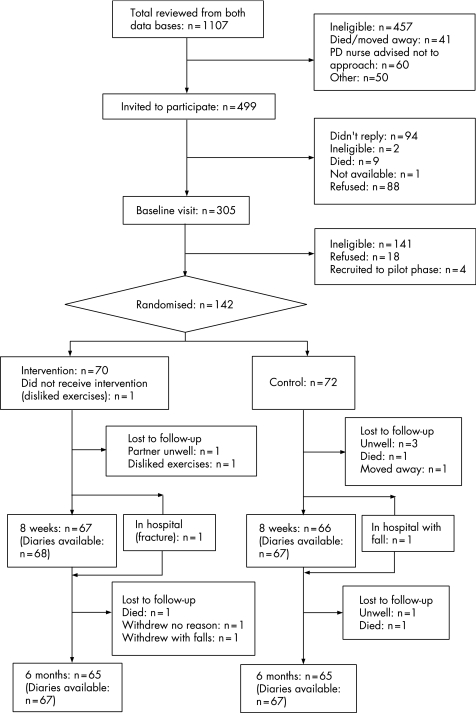
Of the 1107 individuals on the databases who were considered for inclusion in the trial (see fig 1), only 142 (13%) were randomised,25 all of whom had passed the screen for gross cognitive impairment.12 There was good adherence: of the 142 recruited, 9 (6 were controls) were lost to follow‐up at 8 weeks and a further 6 (3 were controls) were lost to follow‐up at 6 months. At 8 weeks, 124/133 (93%) assessments were within the target of 1 week on either side of 56 days. At 6 months, 124/130 (95%) assessments were within the target of 2 weeks on either side of 182 days. With regards to the intervention, 64 of the 70 participants randomised to exercises had six treatment sessions, 5 had seven sessions and 1 had two sessions. At the end of the 8 week assessment, the assessing researcher reported being aware of the allocation for 18 (27%) of the exercise group and 11 (17%) of the controls, the corresponding values at 6 months being 25 (39%) and 14 (22%).
Figure 1 Flow of subjects through the trial assessment.
At baseline, there were no important differences between the groups (table 1). A similar percentage of people in the exercise group (24%) and the control group (22%) received PD related rehabilitation external to the trial, but while the percentage in the exercise group remained similar at each assessment, the percentage in the control group increased from 22% at baseline to 34% at 6 months. Members of the control group were also more likely to be taking dopamine agonists. With respect to recalled falls in the previous year, there was a vast range from 2 to 1820 in the exercise group and 2 to 900 in the control group; the means (60, 61) and medians (6, 5) were similar. Three (4%) individuals in the exercise group and 5 (7%) in the control group reported 365 or more falls in the previous year.
Table 1 Characteristics of participants at baseline.
| Exercise group (n = 70) | Control group (n = 72) | |
|---|---|---|
| Centre | ||
| Christchurch/Bournemouth | 33 (47%) | 34 (47%) |
| Poole | 37 (53%) | 38 (53%) |
| Age (y) | ||
| Mean (SD) | 72.7 (9.6) | 71.6 (8.8) |
| Range | 44–91 | 52–90 |
| Sex | ||
| Male | 38 (54%) | 48 (67%) |
| Female | 32 (46%) | 24 (33%) |
| Time since PD diagnosis (y) | ||
| Mean (SD) | 7.7 (5.8) | 9.0 (5.8) |
| Range | 1–31 | 1–30 |
| No of falls in previous year | ||
| Mean (median) | 60 (6) | 61 (5) |
| Range | 2–1820 | 2–900 |
| No of near falls in previous year* | ||
| Mean (median) | 158 (100) | 193 (100) |
| Range | 0–700 | 0–820 |
| Hoehn and Yahr† | ||
| 2 | 8 (11%) | 8 (11%) |
| 3 | 44 (63%) | 48 (67%) |
| 4 | 18 (26%) | 16 (22%) |
| UPDRS‡ | ||
| Mean (SD) | 19.8 (8.3) | 22.2 (11.9) |
| Range | 3–41 | 4–74 |
| SAS | ||
| Mean (SD) | 56.2 (13.3) | 57.5 (14.1) |
| Range | 36–98 | 36–92 |
| Living status | ||
| Alone | 18 (26%) | 16 (22%) |
| With partner | 43 (61%) | 52 (72%) |
| With family/friends/other | 9 (13%) | 4 (6%) |
| Receiving PD related rehabilitation | 17 (24%) | 16 (22%) |
| Any orthopaedic condition | 48 (69%) | 46 (64%) |
| Any cardiac condition | 30 (43%) | 35 (49%) |
| Any mental health condition | 13/69 (19%) | 20/72 (28%) |
| Taking L‐DOPA plus¶ | 62 (89%) | 57 (79%) |
| Taking dopamine agonists§ | 36 (49%) | 49 (68%) |
PD, Parkinson's disease; SAS, Self‐assessment Parkinson's Disease Disability Scale; UPDRS, Unified Parkinson's Disease Rating Scale.
*Number of near falls was stated as too large to enumerate for one case in each group.
†Hoehn and Yahr: 2 = bilateral/midline involvement, no balance impairment; 3 = impaired righting reactions, mild or moderate disability, capable of leading independent lives; 4 = severe disability, can walk but marked disability on activities of daily living.
‡UPDRS was not available for one control and two exercise group subjects.
¶Madopar or Sinemet.
§Amantadine, apomorphine, bromocriptine, cabergoline, pergolide, pramipexole, ropinerole.
Values are number (%) unless stated otherwise.
Overall there was a consistent trend towards lower fall rates in the exercise group at 8 weeks and at 6 months, and lower injury rates needing medical attention at 6 months, but these reductions did not reach significance (table 2). Diary records by participants on what they thought caused their falls included the following: tripped over feet, legs gave way, turned and overbalanced backwards, lost my balance, stepped backwards and went down. The lower near fall and repeat near fall rates for the exercise group were significant at both 8 weeks and 6 months. To check whether we might have missed a greater reduction in falling among the less severe cases, subgroup differences in falling and repeat falling rates were examined separately for the subgroups with Hoehn and Yahr scores of 2–3 and 4 (table 3). The difference in rates were similar in both severity subgroups at 8 weeks, but at 6 months the exercise group had lower fall rates in the less severe subgroup while in the more severe subgroup the exercise group had higher fall rates. The interaction test was significant (p = 0.021) in the case of repeat falling rates at 6 months, supporting the possibility that a beneficial effect in the less severe subgroup might have been missed.
Table 2 Injuries, single and repeat falling, and near falling rates at 8 weeks and 6 months.
| Exercise group | Control group | Unadjusted exercise−control difference (95% CI) | p Value* | |
|---|---|---|---|---|
| Injuries requiring medical help | ||||
| 6 months (1 or more) | 7/67 (10%) | 11/67 (16%) | −6% (−18%, 6%) | 0.329 |
| 6 months (n) | ||||
| 0 | 60 (90%) | 56 (84%) | 0.282† | |
| 1 | 6 (9%) | 7 (10%) | ||
| 2 | 1 (2%) | 3 (5%) | ||
| 3 | 0 | 1 (2%) | ||
| Fractures | ||||
| 6 months | 2/67 (3%) | 6/67 (9%) | −6% (−3%, 16%) | 0.141 |
| Falling | ||||
| 8 weeks | 37/65 (57%) | 42/64 (66%) | −9% (−25%, 8%) | 0.423 |
| 6 months | 46/63 (73%) | 49/63 (78%) | −5% (−20%, 10%) | 0.645 |
| Repeat falling | ||||
| 8 weeks | 21/65 (32%) | 28/64 (44%) | −11% (−27%, 5%) | 0.245 |
| 6 months | 35/63 (56%) | 42/63 (68%) | −11% (−27%, 6%) | 0.266 |
| Near falling | ||||
| 8 weeks | 46/64 (72%) | 55/63 (87%) | −15% (−30%, −1%) | 0.020 |
| 6 months | 50/62 (81%) | 57/62 (92%) | −11% (−24%, 1%) | 0.048 |
| Repeat near falling | ||||
| 8 weeks | 35/64 (55%) | 49/63 (78%) | −23% (−36%, −7%) | 0.004 |
| 6 months | 40/62 (65%) | 53/62 (86%) | −21% (−35%, −6%) | 0.007 |
| More than 10 near falls | ||||
| 8 weeks | 17/64 (27%) | 17/63 (27%) | 0% (−16%, 15%) | 0.899 |
| 6 months | 23/62 (37%) | 36/62 (58%) | −21% (−37%, −3%) | 0.026 |
*Likelihood ratio test from logistic regression adjusted for SAS at baseline and centre, falling/near falling rates additionally adjusted for number of falls/near falls in previous year at baseline.
†Unadjusted Mann–Whitney U test.
Table 3 Subgroup analyses of single and repeat falling at 8 weeks and 6 months.
| Exercise group | Control group | Unadjusted exercise−control difference (95% CI) | p Value* | |
|---|---|---|---|---|
| Falling | ||||
| 8 weeks | ||||
| All cases | 37/65 (57%) | 42/64 (66%) | −9% (−25%, 8%) | 0.423 |
| H&Y2–3 | 24/48 (50%) | 30/50 (60%) | −10% (−29%, 10%) | 0.350 |
| H&Y4 | 13/17 (77%) | 12/14 (86%) | −9% (−38%, 21%)† | 0.972 |
| Falling | ||||
| 6 months | ||||
| All cases | 46/63 (73%) | 49/63 (78%) | −5% (−20%, 10%) | 0.645 |
| H&Y2–3 | 31/47 (66%) | 37/49 (76%) | −10% (−27%, 9%) | 0.334 |
| H&Y4 | 15/16 (94%) | 12/14 (86%) | 8% (−18%, 37%)† | 0.279 |
| Repeat falling | ||||
| 8 weeks | ||||
| All cases | 21/65 (32%) | 28/64 (44%) | −11% (−27%, 5%) | 0.245 |
| H&Y2–3 | 11/48 (23%) | 18/50 (36%) | −13% (−30%, 5%) | 0.128 |
| H&Y4 | 10/17 (59%) | 10/14 (71%) | −13% (−45%, 22%)† | 0.542 |
| Repeat falling | ||||
| 6 months | ||||
| All cases | 35/63 (56%) | 42/63 (68%) | −11% (−27%, 6%) | 0.266 |
| H&Y2–3 | 20/47 (43%) | 31/49 (63%) | −21% (−39%, −1%) | 0.046 |
| H&Y4 | 15/16 (94%) | 11/14 (79%) | 16% (−12%, 44%)† | 0.041 |
H&Y, Hoehn and Yahr; SAS, Self‐assessment Parkinson's Disease Disability Scale.
*Likelihood ratio test from logistic regression adjusted for number of falls/near falls in the previous year at baseline, SAS at baseline and centre.
†Exact confidence interval.
No significant differences were found between the groups on the Berg Balance Test or the SAS (table 4), or for the timed up and go test, the chair stand test, muscle strength or ankle range of movement (not shown). In contrast, the difference in the Functional Reach Test was significant at 6 months (p = 0.009) (table 4). The distinguishing feature here was that while subjects in the exercises group maintained their ability to reach forward, subjects in the control group deteriorated over the 6 month follow‐up period. A pattern of deterioration in the quality of life of people in the control group was also evident. Participants in the exercises group maintained their perception of quality of life while participants in the control group scored worse over time: the difference between the groups was significant at 6 months (p = 0.033).
Table 4 Balance, SAS and QoL thermometer at 8 weeks and 6 months.
| Exercise group | Control group | Adjusted* exercise−control difference (95% CI) | p Value† | |
|---|---|---|---|---|
| Berg Balance | ||||
| Baseline | 44.3 (9.8) (n = 70) | 43.6 (10.5) (n = 72) | ||
| 8 weeks | 45.8 (9.2) (n = 67) | 45.2 (9.9) (n = 66) | 0.1 (−0.26, 2.25) | 0.120 |
| 6 months | 45.3 (10.0) (n = 64) | 44.6 (11.0) (n = 64) | 0.1 (−1.8, 2.0) | 0.913 |
| Functional Reach | ||||
| Baseline | 23.2 (6.7) (n = 70) | 25.0 (7.0) (n = 71) | ||
| 8 weeks | 23.6 (6.4) (n = 67) | 24.0 (7.0) (n = 66) | 1.2 (−0.3, 2.6) | 0.108 |
| 6 months | 23.8 (6.8) (n = 64) | 22.5 (6.8) (n = 64) | 2.0 (0.5, 3.5) | 0.009 |
| SAS | ||||
| Baseline | 56.2 (13.3) (n = 70) | 57.5 (14.1) (n = 72) | ||
| 8 weeks | 57.2 (14.9) (n = 67) | 57.5 (14.8) (n = 66) | −0.0 (−2.9, 2.8) | 0.980 |
| 6 months | 58.9 (15.4) (n = 64) | 60.5 (15.8) (n = 65) | −0.9 (−4.2, 2.3) | 0.568 |
| QoL thermometer | ||||
| Baseline | 63.1 (17.1) (n = 70) | 64.6 (14.5) (n = 71) | ||
| 8 weeks | 61.3 (19.8) (n = 67) | 61.7 (14.5) (n = 66) | −0.7 (−5.6, 4.3) | 0.793 |
| 6 months | 63.0 (18.7) (n = 65) | 56.6 (16.9) (n = 64) | 5.7 (0.47, 11.0) | 0.033 |
SAS, Self‐assessment Parkinson's Disease Disability Scale; QoL, quality of life.
*Adjusted for SAS at baseline, baseline Berg Balance/Functional Reach/QoL thermometer and centre.
†F test from linear regression adjusted for SAS at baseline, baseline Berg Balance/Functional Reach/QoL thermometer and centre.
Values are mean (SD).
Discussion
This was the first large trial to evaluate the effectiveness of a home based exercise and strategy programme for people with PD who repeatedly fall. Findings from the trial showed a consistent trend of reduced rates of falls and injurious falls among participants in the exercise programme but the differences were not significant. However, rates of near falls and repeat near falling among those in the exercise group were significantly less than those in the control group.
The trend in fall reduction suggests that individuals in the exercise group may have benefited from the exercises in the programme and from following the strategies for safe functional mobility by allowing them to use appropriate balance reactions to save themselves, avoiding near fall situations and minimising the severity of fall injuries. Such benefits have been found by other researchers who have evaluated exercise programmes for reducing falls among the general older population.7,8,9,10,11 Few researchers have reported on near falls which are occasions when an individual manages to save him/herself from falling to a lower level. Following an exercise and awareness programme for older individuals (over 50 years) from the general population, Steinberg et al15 reported a significant reduction in falls and near falls. The findings from the present study and from Steinberg's suggest that individuals may have improved balance control, adaptive saving reactions and the effective use of fall prevention strategies as a result of the intervention programmes, and were able to save themselves from some but not all postural disturbances. Steinberg et al also suggested that as near falls can be considered a precursor to falls, reducing near falls is an important contribution to falls prevention.
Why these strategies failed to produce a significant reduction in fall frequency among the PD population is not totally clear. One possible explanation is that we were unable to recruit our target of 200 subjects. On average, subjects in the study had suffered from PD for 8–9 years, experienced multiple falls, multiple pathologies and took multiple medications. It became evident that changing the movement patterns and behaviour of some subjects in the group who fell daily was extremely challenging. It is possible that single fallers and those with less severe disease severity may be more receptive to changing their movement patterns and behaviour through exercise programmes and training. The results of a subgroup analysis of fall frequency according to disease severity reinforced this point as subjects with less disease severity (Hoehn and Yahr grades 2–3) demonstrated a trend of reduced rates of repeat falling in the exercise group at 8 weeks (p = 0.128) and at 6 months (p = 0.046); a corresponding reduction was not consistently maintained for those with more severe disease (Hoehn and Yahr grade 4). The lack of effect of the intervention on most of the secondary outcomes was surprising but not totally dissimilar to the findings of other researchers9,10 who, despite demonstrating a reduction in fall rate, found a lack of effect on a number of outcomes, including muscle strengthening. This could be explained by the difficulty in assessing muscle strength in the home, but also supports the possibility that the beneficial effect of the intervention was as much related to education and greater confidence as to physical change.11
Trial limitations include the increasing numbers of control subjects who accessed rehabilitation outside of the trial by 6 months, in preference to waiting for advice at the end of the trial. At baseline there were approximately equal numbers of people in the exercise and control groups (24% and 22%, respectively) receiving rehabilitation. These percentages reflect those reported nationally26 and indicate how few people in the UK have PD rehabilitation. By 6 months, 34% of the control group were participating in extra rehabilitation compared with 25% in the exercise group. Involvement in the trial may have raised the interest of participants in fall management and despite being encouraged not to alter their management and being told they would receive advice at the end of the trial, many control subjects chose to seek rehabilitation. To have stated “no involvement in rehabilitation for 6 months”, as an inclusion criterion, we believe would have negatively influenced recruitment and posed ethical problems.
Although we had only one treating physiotherapist in the trial, considerable care was taken to ensure that the intervention programme in the trial reflected evidence based practice: the package of progressive exercises was compiled from the literature and from expert views. The content of each individualised programme and the frequency of practice were carefully documented. Treatment sessions generally lasted for 6 weeks but those with a severe progressive condition may have benefited from more prolonged intervention. Additional support to encourage continued adherence to exercises and strategies following a period of contact with the physiotherapist may also have proved helpful. We chose instead to adopt a pragmatic approach and modelled our intervention within the constraints likely to be experienced in routine practice. The exercise programme developed for the trial was safe when delivered by a physiotherapist; no individual fell while doing their exercises.
Self‐report of fall events remains a vital source of information about people living in the community. Prospective (use of monthly diaries) and retrospective recall are both self‐reported, and both methods were used in this trial, but with differing periods of recall.27 In line with other researchers, we favoured the use of prospective monthly diaries during the trial period10 but as participants were unknown to us prior to recruitment, retrospective recall of fall events (through a face to face questionnaire developed in a previous study28) was essential for identifying those who met the inclusion criteria (two or more previous falls) and for characterising the sample at baseline. Interestingly, Mackenzie et al27 propose that retrospective recall is likely to be less accurate than prospective because of under reporting which suggests that in reality, the fall frequency among a community sample of people with PD may be even higher than the values we have reported.
Conclusion
There was a trend for people with PD in this trial to experience lower rates of falling when receiving home based exercises and strategies for safe functional mobility delivered by a physiotherapist. Significantly fewer people in the exercise group experienced near falls or repeated near falls. The pattern of falling experienced individually was varied and in some cases reached extreme frequency. Subgroup findings suggested that subjects with less severe PD benefited from the exercise programme to a greater extent. Further trials offering this type of intervention earlier in the disease progression (before severe balance problems manifest themselves) are needed.
Acknowledgements
We wish to thank Drs Amar, Harries Jones and Ellis for giving us permission to work with PD Nurses Cindy Thompson, Pam Gillibrand and Alison Bush to identify those people with PD who could be invited to participate in the trial. We are extremely grateful to everyone with PD who participated.
Abbreviations
PD - Parkinson's disease
SAS - Self‐assessment Parkinson's Disease Disability Scale
Footnotes
Funded by: Action Medical Research, John and Lucille Van Geest Foundation.
Competing interests: None.
References
- 1.Campbell A J, Robertson M C, Gardener M M. Elderly people who fall: identifying and managing the causes. Br J Hosp Med 199554520–523. [PubMed] [Google Scholar]
- 2.Ashburn A, Stack E, Pickering R.et al A community dwelling sample of people with Parkinson's disease: characteristics of fallers and non‐fallers. Age Ageing 20013047–52. [DOI] [PubMed] [Google Scholar]
- 3.Ashburn A, Stack E, Pickering R.et al Predicting fallers in a community‐based sample of people with Parkinson's disease. Gerontology 200147277–281. [DOI] [PubMed] [Google Scholar]
- 4.Johnell O, Melton J, Atkinson E, et al. Fracture risk in patients with Parkinsonism: A population‐based study in Olmsted County, Minnesota. Age Ageing 19922132–38. [DOI] [PubMed] [Google Scholar]
- 5.Deane K H O, Jones D, Clarke C E.et al Physiotherapy for patients with Parkinson's disease. Cochrane Database Systematic Reviews 3 2001a; CD002817 [DOI] [PubMed]
- 6.Deane K H O, Jones D, Ellis‐Hill C.et al A comparison of physiotherapy techniques for patients with Parkinson's disease. Cochrane Database Systematic Reviews Issue 1. Chichester: Wiley InterScience, 2001;CD002815 [DOI] [PubMed]
- 7.Gillespie L D, Gillespie W J, Robertson M C.et al Interventions for preventing falls in elderly people. Cochrane Database Systematic Reviews 2005. Issue 4. Chichester: Wiley InterScience, 2005 [DOI] [PubMed]
- 8.Chang J T, Morton S C, Mojica W A.et al Interventions for the prevention of falls in older adults: systematic review and meta‐analysis of randomised clinical trials. BMJ 2004328680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tinetti M, Baker D, McAvay G.et al A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med 1994331821–827. [DOI] [PubMed] [Google Scholar]
- 10.Campbell A J, Robertson M C, Gardiner M M.et al Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ 19973151065–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tinetti M E. Prevention of falls and fall injuries in elderly persons: a research agenda. Prev Med 199423756–762. [DOI] [PubMed] [Google Scholar]
- 12.Folstein M F, Folstein S E, McHugh P R. Mini‐Mental State. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 197512189–198. [DOI] [PubMed] [Google Scholar]
- 13.Clark R, Lord S, Webster I. Clinical parameters associated with falls in an elderly population. Gerontology 199339117–123. [DOI] [PubMed] [Google Scholar]
- 14.Stack E, Ashburn A. Fall events described by people with Parkinson's disease: implications for clinical interviewing and research agenda. Physiother Res Int 19994190–200. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg M, Cartwright C, Peel N.et al A sustainable programme to prevent falls and near falls in community dwelling older people: results of a randomised trial. J Epidemiol Community Health 200054227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connell B, Wolf S for F I C S I T. Environmental and behavioural circumstances associated with falls at home among healthy elderly individuals. Arch Phys Med Rehab 199778179–186. [DOI] [PubMed] [Google Scholar]
- 17.Hoehn M, Yahr M. Parkinsonism; onset, progression and mortality. Neurology 196717427–442. [DOI] [PubMed] [Google Scholar]
- 18.Lang A, Fahn S. Assessment of Parkinson's disease. In: Munsat T, ed. Quantification of neurological deficit. Stoneham MA: Butterworth, 1989285–309.
- 19.Duncan P, Weiner D, Chandler J.et al Functional reach: A new clinical measure of balance. J Gerontol 199045M192–M197. [DOI] [PubMed] [Google Scholar]
- 20.Berg K, Wood‐Dauphinee S, Williams J.et al Measuring balance in the elderly; preliminary development of an instrument. Physiother Can 198941304–311. [Google Scholar]
- 21.Podsiadlo D, Richardson S. The timed up and go: a test of basic functional mobilty for elderly persons. J Am Geriatr Soc 199139141–148. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik J M, Simonsick E M, Ferrucci L.et al A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol Med Sci 199449M85–M94. [DOI] [PubMed] [Google Scholar]
- 23.Brown R, MacCarthey B, Jahanshai M.et al Accuracy of self‐reported disability in patients with Parkinson's disease. Arch Neurol 198946955–959. [DOI] [PubMed] [Google Scholar]
- 24.EuroQuol Group EuroQuol—a new facility for the measurement of health related quality of life. Health Policy 199616199–208. [DOI] [PubMed] [Google Scholar]
- 25.Ashburn A, Pickering R, Fazakarley L.et al Recruitment to a clinical trial from the data bases of specialists in Parkinson's disease. Parkinsonism Relat Disord 20071335–39. [DOI] [PubMed] [Google Scholar]
- 26.Yarrow S.Survey of members of the parkinson's disease society. London: Parkinson's Disease Society, 1999
- 27.Mackenzie L, Byles J, D'Este C. Validation of self‐reported fall events in intervention studies. Clin Rehab 200620331–339. [DOI] [PubMed] [Google Scholar]
- 28.Stack E, Ashburn A. Fall events described by people with Parkinson's disease; implications for clinical interviewing and research agenda. Physiother Res Int 19994190–200. [DOI] [PubMed] [Google Scholar]