Abstract
Dysfunctions of the autonomic nervous system (ANS) are common in Parkinson's disease (PD). Regarding motor disability, deep brain stimulation of the subthalamic nucleus (STN) is an effective treatment option in long lasting PD. The aims of this study were to examine whether STN stimulation has an influence on functions of the ANS and to compare these effects to those induced by levodopa.
Blood pressure (BP) and heart rate (HR) during rest and orthostatic conditions, HR variability (HRV) and breathing‐induced cutaneous sympathetic vasoconstriction (CVC) were tested in 14 PD patients treated with STN stimulation during “ON” and “OFF” condition of the stimulator. The effects of a single dose of levodopa on ANS were tested in 15 PD patients without DBS.
STN stimulation had no influence on cardiovascular ANS functions, whereas CVC was significantly increased. In contrast, levodopa significantly lowered BP and HR at rest and enhanced orthostatic hypotension. Further, HRV, skin perfusion and temperature increased after administration of levodopa.
Our results suggest that in contrast to levodopa, STN stimulation has only minor effects on autonomic functions. Since less pharmacotherapy is needed after STN stimulation, reduced levodopa intake results in relative improvement of autonomic function in deep brain stimulated PD patients.
Autonomic dysfunction is common in Parkinson's disease (PD).1 In common with other drugs, levodopa alters cardiovascular reflexes.2,3,4,5,6 Deep brain stimulation of the subthalamic nucleus (STN‐DBS) is an effective treatment for motor disability in patients with PD of long duration.7 As the basal ganglia are connected to areas involved in regulation of the autonomic nervous system (ANS),8 STN‐DBS may affect ANS functions.
The aim of this study was to investigate the influence of STN‐DBS on ANS compared with the effects of levodopa administration.
Methods
Patients
Fourteen patients with PD (mean age 57.7 (2.8) years; 10 males, 4 females), treated with bilateral STN‐DBS (Medtronic 3389, Minneapolis, Minnesota, USA), and 15 non‐stimulated patients with PD (LD group; mean age 63.4 (2.1) years; 6 males, 8 females), were examined clinically and with autonomic tests. The DBS group remained on their everyday antiparkinson medication and a 30 minute interval was given between turning on/off the stimulator and examination (ONstim, OFFstim).
Except for measurement of skin temperature (see methods), the LD group was investigated after at least 12 h off all antiparkinson medication (OFFMed) and again approximately 30 min after administration of oral soluble levodopa and benserazide (ONMed). The dose of levodopa depended on the patient's regular morning dose of levodopa and was 150–300 mg.
Examinations were in agreement with the local ethics committee. All subjects gave written informed consent.
Autonomic tests
Tests were performed in a quiet environment with a constant room temperature of 24°C.
Heart rate variability
Heart rate variability (HRV) was examined using computer assisted equipment (ProSciCard III; MediSyst GmbH, Germany) at rest and during controlled deep breathing (6 respiratory cycles per minute). The coefficient of variation, root mean square of successive differences, mean circular resultant, expiration–inspiration difference and expiration–inspiration ratio (E/I ratio), as well as a spectral analysis of HRV, were quantified and compared with the normal range of 120 age related healthy subjects.9
Tilt test
Blood pressure (BP) and heart rate (HR) were monitored after 10 min of supine rest on a tilt table. Patients were then moved to the erect position (65°) and BP and HR changes recorded at 1, 3 and 5 min of head‐up tilt. A decrease in systolic BP >20 mm Hg and diastolic BP >10 mm Hg within 3 min of tilting was regarded as orthostatic hypotension.10
Cutaneous sympathetic vasoconstriction and skin temperature
Cutaneous sympathetic vasoconstriction was investigated with patients lying in the supine position using non‐invasive laser Doppler flowmetry (PeriFlux 5000; Perimed AB, Stockholm, Sweden) on the index finger of the non‐tremor dominant hand. Cutaneous blood flow (in perfusion units, Pu) during rest and after five deep inspirations was recorded and the reduction in blood flow calculated as follows: (baseline flow−minimal flow after inspiration)/baseline ×100. The mean decrease was determined averaging the three measurements with the highest decrease in blood flow.
Skin temperature in the DBS group was assessed at the tip of the little finger with an infrared thermometer. In the LD group, skin temperature was assessed continuously for the whole period of the examination, with small loggers affixed to one finger that measured temperature every 6 min (Kooltrak, Geisenheim, Germany).
Statistics
The Wilcoxon signed rank test, U test and Spearman's rank test were used, and p<0.05 was considered statistically significant. Data are presented as mean (SEM).
Results
For the DBS group, mean time after surgery was 17.2 (4.3) months (mean stimulation parameters 3.1 (0.2) volts, 147.5 (6) Hz on both sides). Motor disability, as measured using the Unified Parkinson's Disease Rating Scale III, was significantly reduced by STN‐DBS (26.9 (5.1) vs 11.9 (1.9); p<0.05) and by administration of levodopa (43.1 (3.6) vs 18.8 (2.4); p<0.05).
No differences were found for disease duration or number of drugs with potential side effects on the ANS between the two groups. The dose of levodopa and the effective dose equivalents of antiparkinson drugs were lower in the DBS group compared with the LD group (558.9 (86.4) vs 926.7 (88.8) mg/24 h; p<0.05).
Heart rate variability
Because of increased tremor or rigidity during the OFF condition, HRV could not be examined in all patients. HRV parameters did not differ between OFFstim and ONstim during both resting conditions and deep breathing in the entire group (n = 11). The results of HRV parameters in the LD group are shown in table 1.
Table 1 Heart rate variability parameters in the LD group.
| Parameter | ONMed | OFFMed | p Value |
|---|---|---|---|
| At rest (n = 14) | |||
| VC (%) | 3.5 (0.3) | 3.6 (0.4) | NS |
| RMSSD (ms) | 22.4 (2.3) | 18.8 (2.3) | <0.05 |
| During deep breathing (n = 11) | |||
| VC (%) | 8.3 (1.9) | 4.4 (0.8) | <0.01 |
| RMSSD (ms) | 39.1 (6.2) | 22.3 (4.5) | <0.01 |
| MCR | 0.03 (0.01) | 0.02 (0) | <0.01 |
| E–I | 446.7 (67.1) | 184.7 (52.8) | <0.01 |
| E/I ratio | 1.9 (0.2) | 1.3 (0.1) | <0.01 |
E–I, expiration–inspiration difference; E/I ratio, expiration–inspiration ratio; MCR, mean circular resultant; RMSSD, root mean square of successive differences; VC, coefficient of variation.
All data are presented as mean (SEM).
No correlation between the amount of levodopa administered, daily levodopa dose or duration of disease and HRV parameters was observed.
Tilt test
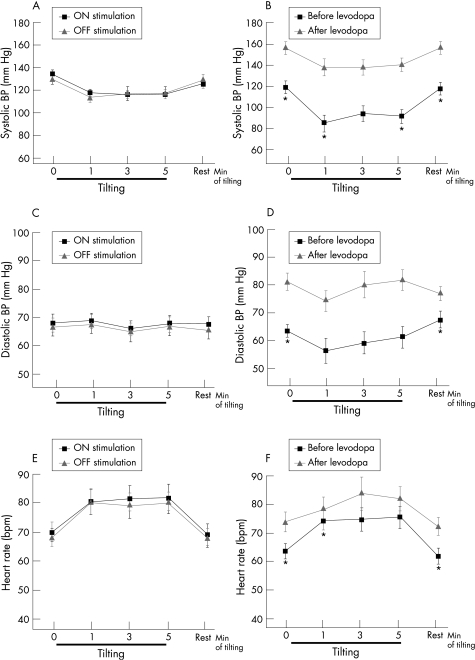
In the STN‐DBS group, three patients showed orthostatic hypotension during ONstim, two in OFFstim and three during both conditions. No significant differences in BP or HR between ONstim and OFFstim were observed. There was no correlation between the decrease in BP in OFFstim and ONstim or between the BP response and motor response to stimulation, age or daily levodopa dose (fig 1).
Figure 1 Blood pressure (BP) and heart rate (HR) at rest and during tilting. (A, C, E) Systolic (A) and diastolic (C) BP and HR (E) during ONstim and OFFstim (mean (SEM)). (B, D, F) Systolic (B) and diastolic (D) BP and HR (F) before and after administration of levodopa (mean (SEM)). *p<0.05 for comparison between ON and OFF.
In the LD group, four patients showed orthostatic hypotension in ONMed and 7 in both conditions. Mean systolic, diastolic, mean arterial BP and HR at rest were significantly lower, and mean systolic BP was significantly more decreased 1 min after tilting in ONMed compared with OFFMed (fig 1). No correlations between duration of disease or amount of soluble levodopa applied and values of BP or HR at rest or during tilting were found.
Cutaneous sympathetic vasoconstriction and skin temperature
Cutaneous vasoconstriction was increased during ONstim compared with OFFstim (−57.2 (3.7) vs −44.8 (5.8)%; p<0.05) whereas blood flow at rest and skin temperature did not differ.
In the LD group, cutaneous vasoconstriction did not differ significantly between ONMed and OFFMed (−34.9 (3.6) vs −40.4 (3.9)%; NS) but was out of the 95% confidence interval during ONMed, according to Schüller et al.11 Skin temperature started to increase approximately 30 min after administration of levodopa and was significantly higher 60 min (32.6 (0.6)°C), 66 min (33 (0.7)°C), 72 min (33.5 (0.4)°C) and 78 min (34 (0.4)°C) after administration of levodopa compared with the baseline value (29.5 (1.3)°C; p<0.05). At this time, skin perfusion at rest was higher in ONMed compared with OFFMed (298 (20.6) vs 179.2 (38.5) Pu; p<0.05). No correlation between dose of levodopa administered and blood flow at rest, skin temperature or cutaneous vasoconstriction was observed.
In both groups, no correlation was observed between disease duration or daily levodopa dose and blood flow at rest, skin temperature or cutaneous sympathetic vasoconstriction.
Discussion
STN‐DBS significantly decreased motor disability but had no direct effect on autonomic cardiac innervation or muscle vasoconstrictor neurons.
Our results are in agreement with other studies that did not find changes in BP or its responses to tilting when examining stimulated patients with PD on 2 consecutive days during ONstim and OFFstim12 or before and 1 year after initiation of STN‐DBS.13
Interestingly, STN‐DBS in patients with PD during surgery increased HR and BP.14 Different findings between intraoperative and post‐surgery investigations could be explained by slightly different intraoperative locations of the STN electrodes,15,16 short lasting alterations in autonomic functions17 or psychological factors (eg, patients are more excited during surgery).
Cutaneous sympathetic vasoconstriction was enhanced in ONstimcompared with OFFstim. Is it possible that STN‐DBS improves selective autonomic functions? For the sympathetic nervous system it has been shown that innervation to the different target organs is controlled separately.18 STN‐DBS acts on fibres and cell bodies close to the implanted electrode.19 Thus an effect of STN‐DBS on specific autonomic pathways may be possible. Accordingly, STN‐DBS has been shown to improve sympathetic skin responses12 and neural regulation of the bladder.20
In contrast with STN‐DBS, levodopa significantly lowered BP and HR at rest. Furthermore, we found a positive correlation between the decrease in BP in OFFMed and ONMed, suggesting that in patients with orthostatic hypotension, levodopa even worsened autonomic dysregulation.
Skin temperature, skin perfusion and HRV were increased, and cutaneous sympathetic vasoconstriction decreased in ONMed. These effects could be explained by a decreased central sympathetic outflow caused by the central D2 agonist action of levodopa.21 Peripherally mediated vasodilation in the vascular bed of skeletal muscle and skin4,5 is unlikely as it would have induced a baroreceptor reflex mediated increase in HR, which was not observed, despite an intact baroreceptor reflex, as indicated by a rise in HR during tilt table testing.
In conclusion, our results suggest that STN‐DBS has some selective positive effects on autonomic functions without any effect on the cardiovascular ANS. In contrast, levodopa induced several negative effects on autonomic functions by lowering BP and HR as well as cutaneous vasodilation, all contributing to worsening orthostatic hypotension. As treatment with STN‐DBS allows reduction of the daily dose of levodopa,19 reduced levodopa intake may result in relative improvement of autonomic function in deep brain stimulated patients with PD.
Abbreviations
ANS - autonomic nervous system
BP - blood pressure
E/I ratio - expiration–inspiration ratio
HR - heart rate
HRV - heart rate variability
PD - Parkinson's disease
STN‐DBS - deep brain stimulation of the subthalamic nucleus
Footnotes
This work was supported by the Bundesministerium für Bildung und Forschung (BMBF, 01EM05/04), der Deutschen Forschungsgemeinschaft (DFG Ba1921/1‐1/3) and der Alexander‐von‐Humboldt‐Stiftung and Pfizer Deutschland (unrestricted educational grant). None of the sponsors was involved in the design or conduct of the study, or in the collection, management, analysis, interpretation or preparation of the data, or review or approval of the manuscript.
Competing interests: None.
References
- 1.Jost W H. Autonomic dysfunctions in idiopathic Parkinson's disease. J Neurol 2003250(Suppl 1)I28–I30. [DOI] [PubMed] [Google Scholar]
- 2.Saito I, Kawabe H, Hasegawa C.et al Effect of L‐dopa in young patients with hypertension. Angiology 199142691–695. [DOI] [PubMed] [Google Scholar]
- 3.Reid J L, Dollery C T. Central and peripheral catecholamine mechanisms in circulatory control. Cardiology 197661(Suppl 1)113–124. [DOI] [PubMed] [Google Scholar]
- 4.Quevedo M, Prieto J C, Perez‐Olea J. The beta 2 receptor plays a role in the hypotensive effect of L‐dopa. Acta Physiol Pharmacol Ther Latinoam 19954519–25. [PubMed] [Google Scholar]
- 5.Quevedo M, Prieto J C, Miranda H F.et al Isobolographic analysis of the interaction between fenoldopam and levodopa on arterial blood pressure of the rat. J Cardiovasc Pharmacol 200036413–415. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi S, Sugiyama Y, Mano T.et al Effect of L‐dopa on human muscle sympathetic nerve activity. Environ Med 19933799–102. [PubMed] [Google Scholar]
- 7.Deuschl G, Schade‐Brittinger C, Krack P.et al A randomized trial of deep‐brain stimulation for Parkinson's disease. N Engl J Med 2006355896–908. [DOI] [PubMed] [Google Scholar]
- 8.Braak H, Braak E. Pathoanatomy of Parkinson's disease. J Neurol 2000247(Suppl 2)II3–NaN10. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler D, Laux G, Dannehl K.et al Assessment of cardiovascular autonomic function: age‐related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabet Med 19929166–175. [DOI] [PubMed] [Google Scholar]
- 10. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. J Neurol Sci 1996144218–219. [PubMed] [Google Scholar]
- 11.Schüller T B, Hermann K, Baron R. Quantitative assessment and correlation of sympathetic, parasympathetic, and afferent small fiber function in peripheral neuropathy. J Neurol 2000247267–272. [DOI] [PubMed] [Google Scholar]
- 12.Priori A, Cinnante C, Genitrini S.et al Non‐motor effects of deep brain stimulation of the subthalamic nucleus in Parkinson's disease: preliminary physiological results. Neurol Sci 20012285–86. [DOI] [PubMed] [Google Scholar]
- 13.Holmberg B, Corneliusson O, Elam M. Bilateral stimulation of nucleus subthalamicus in advanced Parkinson's disease: no effects on, and of, autonomic dysfunction. Mov Disord 200520976–981. [DOI] [PubMed] [Google Scholar]
- 14.Thornton J M, Aziz T, Schlugman D.et al Electrical stimulation of the midbrain increases heart rate and arterial blood pressure in awake humans. J Physiol 2002539615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipp A, Tank J, Trottenberg T.et al Sympathetic activation due to deep brain stimulation in the region of the STN. Neurology 200565774–775. [DOI] [PubMed] [Google Scholar]
- 16.Benedetti F, Colloca L, Lanotte M.et al Autonomic and emotional responses to open and hidden stimulations of the human subthalamic region. Brain Res Bull 200463203–211. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann H, Bhattacharya K F, Voustianiouk A.et al Stimulation of the subthalamic nucleus increases heart rate in patients with Parkinson disease. Neurology 2002591657–1658. [DOI] [PubMed] [Google Scholar]
- 18.Janig W, Habler H J. Neurophysiological analysis of target‐related sympathetic pathways—from animal to human: similarities and differences. Acta Physiol Scand 2003177255–274. [DOI] [PubMed] [Google Scholar]
- 19.Volkmann J. Deep brain stimulation for the treatment of Parkinson's disease. J Clin Neurophysiol 2004216–17. [DOI] [PubMed] [Google Scholar]
- 20.Seif C, Herzog J, van der Horst C.et al Effect of subthalamic deep brain stimulation on the function of the urinary bladder. Ann Neurol 200455118–120. [DOI] [PubMed] [Google Scholar]
- 21.Tonkin A L F D. Drugs, chemicals, and toxins that alter autonomic function. In: Mathias CJ, Bannister SR, eds. Autonomic failure. London: Oxford University Press, 1999527–533.