Abstract
Ocular flutter is a rare abnormal eye movement consisting of irregular bursts of to‐and‐fro bidirectional horizontal saccades and is frequently encountered in association with cerebellar symptoms. We present a patient with a probable post‐infectious ocular flutter that exhibited characteristics not previously reported in the literature. Bursts of ocular flutter consisted almost exclusively of initial rightward saccades and were clearly influenced by orbital eye position and the presence of a visual stimulus. The most recent models of saccadic oscillations do not provide an explanation for such atypical features, especially for the systematic directional bias. Based on existing experimental data, we propose that dysfunction of vermal pause neurons in an unstable saccade network could account for such atypical characteristics.
Ocular flutter is an abnormal eye movement consisting of repetitive, irregular, involuntary bursts of horizontal saccades without an intersaccadic interval.1 It is generally superimposed on normal oculomotor behaviour and its occurrence may be favoured by various events, such as blinks, the triggering of normal saccades or optokinetic stimulation.2,3 Its physiopathology remains unclear although it is currently accepted that it probably results from a dysfunction of brainstem oculomotor structures involved in saccade generation: excitatory burst neurons (EBN), inhibitory burst neurons (IBN) and omnipause neurons (OPN) that keep EBN and IBN silent, except immediately before and during saccade execution. Earlier hypotheses ascribed saccadic oscillations to impaired OPN function.4 More recently, instability in positive feedback loops involving EBN and IBN has been hypothesised as the critical factor responsible for saccadic oscillations, as may be observed in normal subjects.5
Here, we report the case of a patient with an atypical ocular flutter not previously described in the literature. The most striking feature was that saccadic oscillations systematically started with a rightward saccade, a characteristic that cannot be explained by current models. In order to account for this systematic directional bias, we propose that dysfunction of vermal pause neurons may be involved in the pathophysiology of ocular flutter, as experimental data have shown that these cells exhibit a marked directional selectivity.
Case report
A 35‐year‐old man with no previous medical history experienced a rapid onset of dizziness, motion induced nausea and blurred vision, 3 weeks after returning from Africa. No previous symptoms of febrile infectious disease were reported. On neurological admission, the patient was able to stand but showed a clear truncal ataxia with a tendency to lean to the right. Gait was unsteady and broad based. There was no motor or sensory deficit, no tremor and no limb ataxia. Cranial nerve examination was normal and deep tendon reflexes were all present. Clinical eye movement examination revealed frequent but irregular involuntary bursts of large to‐and‐fro horizontal saccades, responsible for oscillopsia. These saccades were of larger amplitude and higher frequency when the patient was asked to look to the right. Natural horizontal and vertical saccades were conjugate and of normal amplitude. Ocular motor recording was performed 2 days after admission. Blood count and routine biochemistry were normal. CT and brain MRI performed on admission were normal. CSF contained 7 lymphocytes/mm3 and showed a mild rise in protein count (63 mg/dl). Antineuronal antibodies (anti‐HU, anti‐YO and anti‐RI) were absent. No seroconversion was observed for the main neurotropic viruses. A subsequent brain MRI, performed 10 days after admission, remained normal and, on follow‐up, all symptoms progressively resolved within 6 weeks.
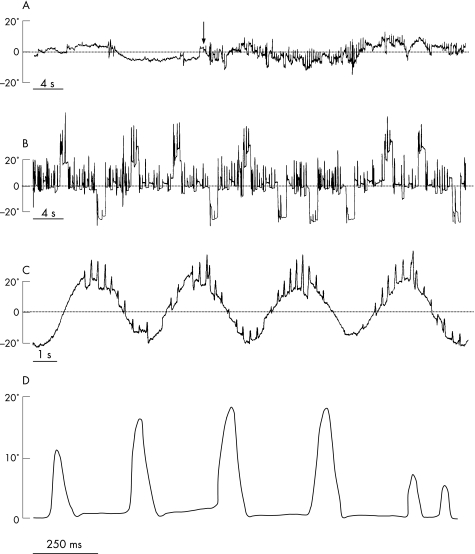
Eye movements were recorded binocularly with DC electro‐oculography, and with an infrared device (Skalar IRIS, Delft, the Netherlands) over the right eye. The patient was seated in complete darkness with the head immobilised. Visual targets were presented on a ramp embedded with LEDs, located 90 cm in front of the subject. Three paradigms were performed: a fixation task, a reflexive saccade task and a smooth pursuit task. In the fixation task, the patient was asked to look at a stationary central target that was switched on and off from time to time. The analysis showed bursts of horizontal to‐and‐fro saccades without an intersaccadic interval, which confirmed the diagnosis of ocular flutter. Saccadic oscillations consisted mostly of two successive saccades in opposite directions and rarely in a succession of more than two saccades. In all cases, these oscillations were initiated by a rightward saccade. The occurrence of these oscillations dramatically decreased when the fixation target was switched off (fig 1A). In the saccade task, the patient was required to follow a target that jumped with unpredictable timing from a central position to a lateral right or left position (either 10° or 25°). A 200 ms gap was interposed between the central target offset and the lateral target onset. Reflexive visually guided saccade latencies were significantly shorter leftward (mean 135 (SD 27) ms) than rightward (mean 168 (47) ms) (Student's t test, p = 0.01). This task further confirmed that, within these saccadic oscillations, saccade frequency and amplitude progressively increased when the eyes were moved from the extreme left to the extreme right orbital position (fig 1B). Whereas the ocular flutter almost disappeared on leftward gaze, its amplitude reached 10–15° and its frequency 2–3 Hz in the central position. On maximum rightward gaze, its amplitude further increased up to 15–25° with a frequency of 4–6 Hz. Peak velocity of 20° oscillations was found between 350 and 400°/s, which corresponds to the values of normal saccades. During smooth pursuit, saccadic oscillations seemed to be superimposed on normal smooth eye movements (fig 1C).
Figure 1 Eye movement recording. (A) Eye movements during fixation of a stationary target. The ocular flutter clearly increased when the target was turned on (vertical arrow). (B) Horizontal visually guided saccades. Note that ocular flutter increased in frequency and amplitude on rightward gaze. (C) Horizontal smooth pursuit. Note that the initial saccade within a saccadic oscillation was systematically directed to the right. Here, an upward deflection corresponds to a rightward eye position. (D) Saccadic oscillations on a large time scale.
Discussion
The horizontal to‐and‐fro saccadic oscillations observed in this patient correspond to the definition of ocular flutter.1 Its association with truncal ataxia and CSF pleocytosis has previously been reported, and its spontaneous resolution is compatible with a post‐infectious syndrome.6 However, this ocular flutter presented atypical characteristics not previously reported. Its most striking features were that it consisted of saccadic oscillations that were all initially triggered rightwards, with a clear influence of intraorbital eye position.
Models of saccade oscillations have included various dysfunctions of brainstem structures. As OPN inhibit saccadic burst neurons, initial hypotheses relied on OPN dysfunction, but have not received support from lesion studies.7 A more recent model has concentrated on positive feedback loops between EBN and IBN. According to this model, adaptive properties of the membranes of these cells would result in a post‐inhibitory rebound of activity, for example, at offset of OPN hyperpolarisation. Consequently, depolarisation in a group of EBN would trigger a saccade, and positive feedback loops between burst neurons on both sides of the brainstem would result in the alternation of bidirectional saccadic pulses (ie, in saccadic oscillations). However, the initial saccadic burst would be randomly directed in one direction. In order to create a systematic directional bias in this unstable network, it may be necessary to consider dysfunction of inhibitory neurons with a clear directional preference. Interestingly, pause neurons with a selective pause of discharge immediately before and during contralateral saccades have been described in the cerebellar vermis.8 It may therefore be hypothesised that selective dysfunction of these pause neurons would create an imbalance between burst neuron excitability on both sides of the brainstem, favouring the initial triggering of unidirectional saccades. Moreover, most saccade related cells in the cerebellar vermis encode the position of the eyes in the orbit,9 and several vermal cell types show significantly reduced activity in the dark.10 Ocular flutter may result from different mechanisms. We propose that, at least in the case of unidirectional flutter, vermal pause neurons may be involved in the pathophysiological process, especially when associated with truncal ataxia.
Abbreviations
EBN - excitatory burst neurons
IBN - inhibitory burst neurons
OPN - omnipause neurons
Footnotes
Competing interests: None.
References
- 1.Leigh J, Zee D S. Diagnosis of nystagmus and saccadic intrusion. In: Leigh RJ, Zee DS, eds. The neurology of eye movements, 4th Edn. Oxford: Oxford University Press, 2006475–558.
- 2.Hain T C, Zee D S, Mordes M. Blink‐induced saccadic oscillations. Ann Neurol 198619299–301. [DOI] [PubMed] [Google Scholar]
- 3.Koh S H, Kim S H. Ocular flutter induced only by optokinetic stimulation. J Clin Neurosci 200613479–481. [DOI] [PubMed] [Google Scholar]
- 4.Zee D S, Robinson D A. A hypothetical explanation of saccadic oscillations. Ann Neurol 19795405–414. [DOI] [PubMed] [Google Scholar]
- 5.Ramat S, Leigh R J, Zee D S.et al Ocular oscillations generated by coupling of brainstem excitatory and inhibitory saccadic burst neurons. Exp Brain Res 200516089–106. [DOI] [PubMed] [Google Scholar]
- 6.Wiest G, Safoschnik G, Schnaberth G.et al Ocular flutter and truncal ataxia may be associated with enterovirus infection. J Neurol 1997244288–292. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko C R. Effect of ibotenic acid lesions of the omnipause neurons on saccadic eye movements in rhesus macaques. J Neurophysiol 1996752229–2242. [DOI] [PubMed] [Google Scholar]
- 8.Ohtsuka K, Noda H. Discharge properties of Purkinje cells in the oculomotor vermis during visually guided saccades in the macaque monkey. J Neurophysiol 1995741828–1840. [DOI] [PubMed] [Google Scholar]
- 9.Quaia C, Lefevre P, Optican L M. Model of the control of saccades by superior colliculus and cerebellum. J Neurophysiol 199982999–1018. [DOI] [PubMed] [Google Scholar]
- 10.Helmchen C, Buttner U. Saccade‐related Purkinje cell activity in the oculomotor vermis during spontaneous eye movements in light and darkness. Exp Brain Res 1995103198–208. [DOI] [PubMed] [Google Scholar]