Abstract
Background
The activity of the immune system displays a circadian rhythm. In diseases characterised by aberrant immune activity, chronotherapy (a treatment regimen tailored to diurnal body rhythms) may increase the efficiency, safety and tolerability of drugs.
Aim
To compare the outcomes of intravenous corticosteroid administration during the day or night, for treatment of acute multiple sclerosis relapses.
Methods
17 patients with multiple sclerosis were included in the study. Clinical assessment of disability was performed at trial entry, and at days 7 and 30 from the initiation of treatment. Adverse events and preference of night‐time versus daytime treatment were assessed at the end of the treatment course.
Results
After night‐time treatment, clinical recovery was significantly (p<0.001) enhanced and the mean number of side effects was significantly (p = 0.007) lower. Furthermore, most patients expressed a preference for night‐time versus daytime treatment.
Conclusions
The study suggests a potential benefit for implementation of chronotherapy using steroid treatment for acute multiple sclerosis relapse, with implications for other immune‐mediated disorders.
Diurnal rhythm is a characteristic of the immune system, displaying periodicity in the activity of both its humoral and cellular arms. Among the recognised humoral mediators that exhibit a circadian pattern is cortisol, a key circulating glucocorticoid with potent endogenous immunosuppressant activity, which imposes diurnal variation on a broad spectrum of immune functions.1 Accordingly, periods of enhanced immune reactivity coincide with or follow the early morning nadir in plasma cortisol. For example, the interferon (IFN)γ:interleukin (IL) 10 (proinflammatory:anti‐inflammatory cytokines) ratio peaks during the early morning and correlates negatively with plasma cortisol levels.2 At the cellular level, diurnal rhythms are also seen in natural killer cell activity, the CD4:CD8 ratio and the absolute number of circulating T cells.3
Recognition of the importance of diurnal patterns has led to the development of the innovative area of chronotherapy, the application of chronobiological principles to therapeutics. Recently, this strategy of chronotherapy has been successfully implemented in therapeutics of asthma, cancer, gastrointestinal and cardiovascular diseases.4,5
Multiple sclerosis, a chronic inflammatory disease of the central nervous system, is associated with seasonal variation of cytokine secretion and lesion activity in affected individuals,6 as well as with diurnal variations in symptoms.7 In the past decade, new horizons have opened in treatments for multiple sclerosis; nonetheless, patients treated with immunomodulators still continue to experience relapses, albeit at a lower rate.8,9,10 These acute multiple sclerosis‐related exacerbations are commonly treated with glucocorticoids, specifically, high‐dose methylprednisolone.11
This study was designed to examine the clinical effect of intravenous corticosteroids administered at night time compared with daytime, for treatment of the acute multiple sclerosis relapse, and presents the possible benefits of the chronotherapy approach for patients with multiple sclerosis.
Methods
Patients
Patients (18–55 years) were recruited through the multiple sclerosis clinic at the Carmel Medical Center, Haifa, Israel. Inclusion criteria were diagnosis of relapsing remitting multiple sclerosis, meeting the clinical criteria of Poser et al.12 Only relapses (defined as given by Galboiz et al13) associated with considerable functional impairment were indicators for treatment and included in the study. Exclusion criteria were a history of steroid resistance, steroid‐intolerance intractable peptic ulcer, diabetes, osteoporosis or acute infection.
Eligible patients with clinical presentation of acute exacerbation were randomly assigned to either daytime (10:00–14:00) or night‐time (22:00–02:00 in a non‐illuminated setting) treatment protocol of drug administration. The protocol of corticosteroid treatment was intravenous methylprednisolone (IVMP) 1 g/day for 3 consecutive days, followed by 0.5 g/day for an additional 3 days. The IVMP course was followed by a subsequent tapering course of oral corticosteroids (prednisone), starting from 1 mg/kg with a reduction of 5 mg every 2 days. Patients who were treated by the night‐time protocol and presented at a successive relapse episode were treated with the daytime protocol, and vice versa. Overall, comparative clinical data were collected from 17 patients; each received daytime and night‐time intravenous treatment for different relapse episodes during the study period. The study was conducted according to the approval of the Carmel Medical Center Ethical Committee.
Clinical assessment
A full clinical assessment, including disability and adverse events, was completed by researchers blinded to the treatment protocol. Assessment of disability using Kurtzke's Expanded Disability Status Scale (EDSS)14 was performed at admission for relapse treatment and compared with prerelapse disability, as well as with disability at days 7 and 30 after initiation of IVMP administration. The severity of the acute episode was assessed by the change in disability according to
ΔEDSSseverity=(EDSS at relapse before treatment)−(EDSS at prior remission)
The change in clinical status after treatment was calculated by
ΔEDSStreatment=(EDSS post‐treatment at day X (7 or 30))−(EDSS at relapse before treatment)
Adverse events were evaluated by patient interview and a specific questionnaire.
The patients' preference for future treatment regimen was assessed at the termination of the study. We used the following 5‐point preference scale: (1) strong preference for daytime treatment; (2) moderate preference for daytime treatment; (3) indifferent; (4) moderate preference for night‐time treatment; and (5) strong preference for night‐time treatment.
Statistical evaluation
Paired comparison of baseline clinical parameters and clinical outcomes after daytime versus night‐time treatments for the same patient was performed using Wilcoxon's signed rank test. The patients' preferences were compared by χ2 test for goodness of fit. For all analyses, p<0.05 were considered significant.
Results
Demographic and clinical characteristics of patients with multiple sclerosis
In all, 17 patients (11 women) enrolled in this study, receiving alternating daytime or night‐time glucocorticoid treatment for consecutive multiple sclerosis relapses. The clinical and demographic baseline parameters of the patients at daytime versus night‐time IVMP treatment were not significantly different, including mean (standard deviation (SD)) age (35.4 (13.3) years in daytime v 35.1 (13.3) years in night‐time treatment groups), disease duration (4.44 (2.9) years in daytime v 4.17 (2.56) years in night‐time treatment groups) and disease activity, measured as the number of steroid courses in the year before the study. Mean (SD) EDSS at the remission period before the relapse episode in both groups was similar (3.88 (1.55) v 3.55 (1.32) in daytime and night‐time treatment groups, respectively). Mean (SD) EDSS at relapse before initiation of treatment was not significantly different for the patients between the daytime and night‐time treatment groups (6.1 (1.4) v 6.5 (1.4), respectively). However, the mean (SD) severity of the acute episode (measured by ΔEDSSseverity) was slightly higher in the night‐time group (ΔEDSSseverity = 2.68 (0.58)) than in the daytime group (ΔEDSSseverity = 2.26 (0.79); p = 0.046). The time from the onset of symptoms of the relapse to initiation of glucocorticoid treatment was similar, with an average of 10 days in both treatment groups.
Clinical improvement after IVMP treatment
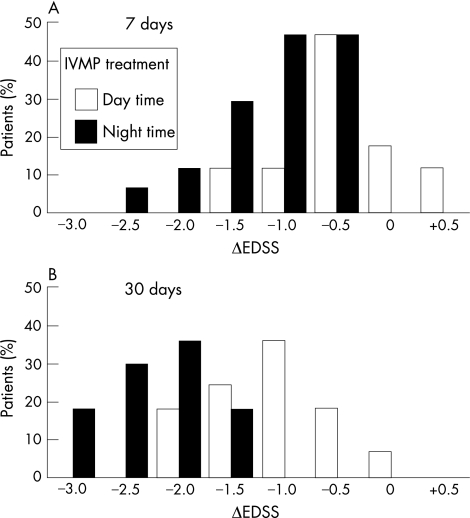
Improvement in the clinical status, manifested as a reduction in the EDSS evaluation (ΔEDSStreatment<0), was observed in both treatment groups after treatment. However, at day 7 after the commencement of treatment, the reduction in EDSS values observed in the night‐time treated group was significantly larger (p<0.001) than those in the daytime‐treated group (fig 1). Only the night‐time group attained a reduction of >2 points in EDSS. The differences between the magnitudes of functional improvement after daytime or night‐time treatments were more pronounced 30 days after the initiation of IVMP treatment (p<0.001). Furthermore, functional improvement seems to occur earlier when glucocorticoids are administered at night.
Figure 1 Functional improvement assessed by reduction of disability after 6 days of intravenous methylprednisolone (IVMP) treatment: ΔEDSStreatment (as defined in the Methods section) at day 7 (A) and day 30 (B). ΔEDSStreatment values were significantly different between daytime‐treated (white bars) and night‐time‐treated (black bars) groups (p<0.001). EDSS, Expanded Disability Status Scale.
Adverse events and patients' preference for treatment mode
For each adverse event considered (table 1), the number of patients who experienced it during the course of a daytime treatment exceeded or was equal to the number of patients who experienced it after night‐time treatment. Overall, the mean number of adverse events per patient was significantly lower after night‐time treatment than after daytime treatment (p = 0.007).
Table 1 Comparison of the number of adverse events experienced by patients receiving daytime or night‐time intravenous methylprednisolone treatment.
| Adverse event | Daytime treatment | Night‐time treatment |
|---|---|---|
| n (%) | n (%) | |
| Restlessness, nervousness | 9 (52.9) | 4 (23.5) |
| Insomnia | 9 (52.9) | 6 (35.3) |
| Dysphoria | 6 (35.3) | 8 (47.1) |
| Depression | 5 (29.4) | 5 (29.4) |
| Headache | 6 (35.3) | 3 (17.6) |
| Palpitations | 7 (41.2) | 3 (17.6) |
| Acne | 2 (11.8) | 2 (11.8) |
| Increased hair growth | 3 (17.6) | 3 (17.6) |
| Unpleasant metallic taste | 10 (58.8) | 6 (35.3) |
| Hot flashes | 8 (47.1) | 3 (17.6) |
| Frequent urination | 10 (58.8) | 9 (52.9) |
| Gastrointestinal symptoms | 1 (5.9) | 2 (11.8) |
| Increased appetite, weight gain | 4 (23.5) | 4 (23.5) |
| Oedema, swelling of feet | 5 (29.4) | 4 (23.5) |
| Musculoskeletal pain | 2 (11.8) | 2 (11.8) |
| Other | 4 (23.5) | 4 (23.5) |
| Mean (SD) number of total adverse events | 5.1 (2.1) | 3.8 (2.3)* |
*p = 0.007.
Most of the patients (10 of 16; 62.5%, excluding 1 patient who was indifferent) expressed a stronger preference for night‐time treatment, with 7 expressing a strong preference. None of the patients expressed a strong preference for daytime treatment. Although the preference for night‐time treatment did not reach statistical significance because of the small group size available for this study, the positive trend seemed firm.
Discussion
This study was designed to assess whether applying chronotherapeutic concepts to corticosteroid treatment in multiple sclerosis may be beneficial to patient care. In this study, clinical improvement was observed earlier, and to a larger extent, in the night‐time treatment group, with a clear effect on day 7, and a more significant effect at 30 days from the initiation of treatment. Treatment tolerability was found to be better after night‐time treatment, as seen by the reduced number of adverse events. Night‐time treatment was expected to lead to disruptions in the circadian rhythm, owing to interrupted sleep. However, this was not an issue raised by the patients. The patients' clear preference for night‐time treatment seems to be a reflection of their subjective perception of the clinical efficacy and experience with adverse events.
To date, various trials of steroids in the treatment of multiple sclerosis have focused on the evaluation of various steroid compounds, dose and/or route of administration.15,16 To our knowledge, this is the first study to show the time‐dependent efficacy and adverse effects of intravenous corticosteroids administered for multiple sclerosis relapse, as part of exploring the possible benefits of using chronotherapy for multiple sclerosis. Our results, although in a relatively small pilot study, suggest that a chronopharmacological approach17,18,19 may be effective, tolerable and, from the patients' subjective perception, a preferential regimen for the treatment of acute relapse of multiple sclerosis. Thus, the use of glucocorticoid chronotherapy should be evaluated in larger and extended controlled clinical trials for multiple sclerosis. Additionally, studies of immune indicators (such as cytokine profile) and blinded assessments of gadolinium‐enhanced magnetic resonance imaging, to confirm the suggestion of a clinical benefit, as well as an assessment of whether the different regimens lead to considerably different outcomes at 6 or 12 months,20 would be important to substantiate the change in clinical management of exacerbation. Notably, special attention should be given to the potential benefit of night‐time glucocorticoid treatment for the subgroup of patients who seem to be resistant to commonly used glucocorticoid protocols21,22 and for whom treatment options at relapse are limited, potentially resulting in rapid clinical deterioration. Owing to the broad usage of corticosteroids as the treatment of choice for immune‐mediated disorders, the development of an efficient protocol for glucocorticoid treatment will have widespread applicability.
Acknowledgements
We thank Sara Dishon and Frida Vardi for assisting in providing patients' care and data management, and Drs Ada Tamir and Ofra Barnett‐Griness for their assistance in statistical analysis.
Abbreviations
EDSS - Expanded Disability Status Scale
IVMP - intravenous methylprednisolone
Footnotes
Funding: This work was supported by funds provided by the Carmel Medical Center, The Rappaport Institute for Research in the Medical Sciences and the Technion–Israel Institute of Technology, Haifa, Israel.
Competing interests: None.
References
- 1.Young M W. The tick‐tock of the biological clock. Sci Am 200028264–71. [DOI] [PubMed] [Google Scholar]
- 2.Petrovsky N, Harrison L C. Diurnal rhythmicity of human cytokine production: a dynamic disequilibrium in T helper cell type 1/T helper cell type 2 balance? J Immunol 19971585163–5168. [PubMed] [Google Scholar]
- 3.Haus E, Smolensky M H. Biologic rhythms in the immune system. Chronobiol Int 199916581–622. [DOI] [PubMed] [Google Scholar]
- 4.Mormont M C, Levi F. Cancer chronotherapy: principles, applications, and perspectives. Cancer 200397155–169. [DOI] [PubMed] [Google Scholar]
- 5.Moor J G. Rhythms and therapeutics of diseases of the gastrointestinal tract. In: Redfern P, ed. Chronotherapeutics. London: Pharmaceutical, 2003127–151.
- 6.Auer D P, Schumann E M, Kumpfel T.et al Seasonal fluctuations of gadolinium‐enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol 200047276–277. [PubMed] [Google Scholar]
- 7.Sandyk R. Diurnal variations in vision and relations to circadian melatonin secretion in multiple sclerosis. Int J Neurosci 1995831–6. [DOI] [PubMed] [Google Scholar]
- 8.Johnson K P, Brooks B R, Cohen J A.et al Copolymer 1 reduces relapse rate and improves disability in relapsing‐remitting multiple sclerosis: results of a phase III multicenter, double‐blind placebo‐controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 1995451268–1276. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs L D, Cookfair D L, Rudick R A.et al Intramuscular interferon beta‐1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 199639285–294. [DOI] [PubMed] [Google Scholar]
- 10.IFNB MS Study Group Interferon beta‐1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. The IFNB Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology 1995451277–1285. [PubMed] [Google Scholar]
- 11.Noseworthy J H, Lucchinetti C, Rodriguez M.et al Multiple sclerosis. N Engl J Med 2000343938–952. [DOI] [PubMed] [Google Scholar]
- 12.Poser C M, Paty D W, Scheinberg L.et al New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 198313227–231. [DOI] [PubMed] [Google Scholar]
- 13.Galboiz Y, Shapiro S, Lahat N.et al Matrix metalloproteinases and their tissue inhibitors as markers of disease subtype and response to interferon‐beta therapy in relapsing and secondary‐progressive multiple sclerosis patients. Ann Neurol 200150443–451. [DOI] [PubMed] [Google Scholar]
- 14.Kurtzke J F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983331444–1452. [DOI] [PubMed] [Google Scholar]
- 15.Sellebjerg F, Frederiksen J L, Nielsen P M.et al Double‐blind, randomized, placebo‐controlled study of oral, high‐dose methylprednisolone in attacks of MS. Neurology 199851529–534. [DOI] [PubMed] [Google Scholar]
- 16.Miller D M, Weinstock‐Guttman B, Bethoux F.et al A meta‐analysis of methylprednisolone in recovery from multiple sclerosis exacerbations. Mult Scler 20006267–273. [DOI] [PubMed] [Google Scholar]
- 17.Smolensky M H, Reinberg A. The chronotherapy of corticosteroids: practical application of chronobiologic findings to nursing. Nurs Clin North Am 197611609–619. [PubMed] [Google Scholar]
- 18.Pincus D J, Humeston T R, Martin R J. Further studies on the chronotherapy of asthma with inhaled steroids: the effect of dosage timing on drug efficacy. J Allergy Clin Immunol 1997100771–774. [DOI] [PubMed] [Google Scholar]
- 19.Ozaydin M, Dede O, Dogan A.et al Effects of morning versus evening intake of atorvastatin on major cardiac event and restenosis rates in patients undergoing first elective percutaneous coronary intervention. Am J Cardiol 20069744–47. [DOI] [PubMed] [Google Scholar]
- 20.Montalban X. Do steroids have a long‐term benefit? In: Thompson AJ, Polman C, Hohlfeld R, eds. Multiple sclerosis: clinical challenges and controversies London: Martin Dunitz, 1997155–167.
- 21.DeRijk R H, Eskandari F, Sternberg E M. Corticosteroid resistance in a subpopulation of multiple sclerosis patients as measured by ex vivo dexamethasone inhibition of LPS induced IL‐6 production. J Neuroimmunol 2004151180–188. [DOI] [PubMed] [Google Scholar]
- 22.van Winsen L M, Muris D F, Polman C H.et al Sensitivity to glucocorticoids is decreased in relapsing remitting multiple sclerosis. J Clin Endocrinol Metab 200590734–740. [DOI] [PubMed] [Google Scholar]